Page 345 - MODERN ELECTROCHEMISTRY
P. 345
ION–ION INTERACTIONS 281
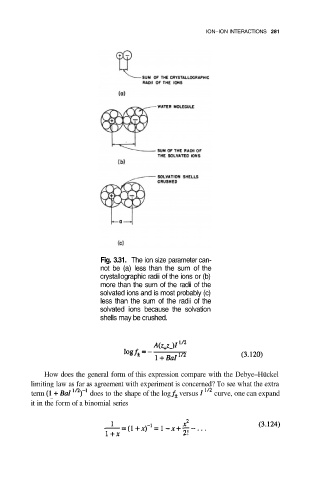
Fig. 3.31. The ion size parameter can-
not be (a) less than the sum of the
crystallographic radii of the ions or (b)
more than the sum of the radii of the
solvated ions and is most probably (c)
less than the sum of the radii of the
solvated ions because the solvation
shells may be crushed.
How does the general form of this expression compare with the Debye–Hückel
limiting law as far as agreement with experiment is concerned? To see what the extra
term does to the shape of the log versus curve, one can expand
it in the form of a binomial series