Page 348 - MODERN ELECTROCHEMISTRY
P. 348
284 CHAPTER 3
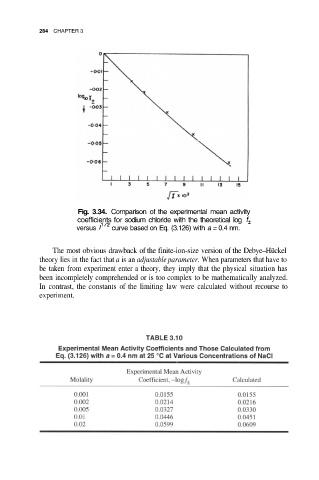
Fig. 3.34. Comparison of the experimental mean activity
coefficients for sodium chloride with the theoretical log
versus curve based on Eq. (3.126) with a = 0.4 nm.
The most obvious drawback of the finite-ion-size version of the Debye–Hückel
theory lies in the fact that a is an adjustable parameter. When parameters that have to
be taken from experiment enter a theory, they imply that the physical situation has
been incompletely comprehended or is too complex to be mathematically analyzed.
In contrast, the constants of the limiting law were calculated without recourse to
experiment.