Page 334 - MODERN ELECTROCHEMISTRY
P. 334
270 CHAPTER 3
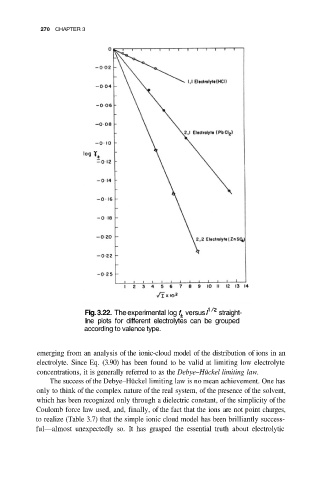
Fig. 3.22. The experimental log versus straight-
line plots for different electrolytes can be grouped
according to valence type.
emerging from an analysis of the ionic-cloud model of the distribution of ions in an
electrolyte. Since Eq. (3.90) has been found to be valid at limiting low electrolyte
concentrations, it is generally referred to as the Debye–Hückel limiting law.
The success of the Debye–Hückel limiting law is no mean achievement. One has
only to think of the complex nature of the real system, of the presence of the solvent,
which has been recognized only through a dielectric constant, of the simplicity of the
Coulomb force law used, and, finally, of the fact that the ions are not point charges,
to realize (Table 3.7) that the simple ionic cloud model has been brilliantly success-
ful—almost unexpectedly so. It has grasped the essential truth about electrolytic