Page 333 - MODERN ELECTROCHEMISTRY
P. 333
ION–ION INTERACTIONS 269
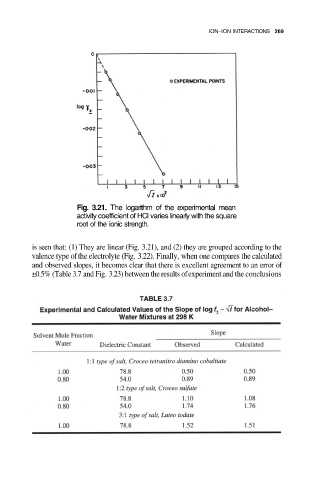
Fig. 3.21. The logarithm of the experimental mean
activity coefficient of HCI varies linearly with the square
root of the ionic strength.
is seen that: (1) They are linear (Fig. 3.21), and (2) they are grouped according to the
valence type of the electrolyte (Fig. 3.22). Finally, when one compares the calculated
and observed slopes, it becomes clear that there is excellent agreement to an error of
±0.5% (Table 3.7 and Fig. 3.23) between the results of experiment and the conclusions