Page 372 - MODERN ELECTROCHEMISTRY
P. 372
308 CHAPTER 3
In this context, Bjerrum took an arbitrary step and cut off the integral at the value
of r = q corresponding to the minimum of the vs. r curve. This minimum probability
can easily be shown (Appendix 3.4) to occur at
Bjerrum argued that it is only short-range Coulombic interactions that lead to
ion-pair formation and, further, when a pair of oppositely charged ions are situated at
a distance apart of r > q, it is more appropriate to consider them free ions.
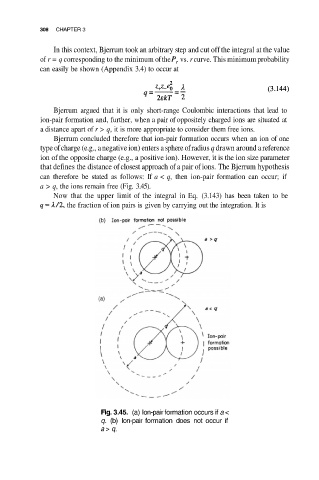
Bjerrum concluded therefore that ion-pair formation occurs when an ion of one
type of charge (e.g., a negative ion) enters a sphere of radius q drawn around a reference
ion of the opposite charge (e.g., a positive ion). However, it is the ion size parameter
that defines the distance of closest approach of a pair of ions. The Bjerrum hypothesis
can therefore be stated as follows: If a < q, then ion-pair formation can occur; if
a > q, the ions remain free (Fig. 3.45).
Now that the upper limit of the integral in Eq. (3.143) has been taken to be
the fraction of ion pairs is given by carrying out the integration. It is
Fig. 3.45. (a) Ion-pair formation occurs if a <
q. (b) Ion-pair formation does not occur if
a > q.