Page 367 - MODERN ELECTROCHEMISTRY
P. 367
ION–ION INTERACTIONS 303
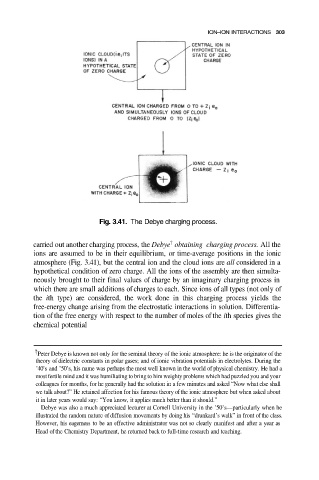
Fig. 3.41. The Debye charging process.
†
carried out another charging process, the Debye obtaining charging process. All the
ions are assumed to be in their equilibrium, or time-average positions in the ionic
atmosphere (Fig. 3.41), but the central ion and the cloud ions are all considered in a
hypothetical condition of zero charge. All the ions of the assembly are then simulta-
neously brought to their final values of charge by an imaginary charging process in
which there are small additions of charges to each. Since ions of all types (not only of
the ith type) are considered, the work done in this charging process yields the
free-energy change arising from the electrostatic interactions in solution. Differentia-
tion of the free energy with respect to the number of moles of the ith species gives the
chemical potential
† Peter Debye is known not only for the seminal theory of the ionic atmosphere: he is the originator of the
theory of dielectric constants in polar gases; and of ionic vibration potentials in electrolytes. During the
’40’s and ’50’s, his name was perhaps the most well known in the world of physical chemistry. He had a
most fertile mind and it was humiliating to bring to him weighty problems which had puzzled you and your
colleagues for months, for he generally had the solution in a few minutes and asked “Now what else shall
we talk about?” He retained affection for his famous theory of the ionic atmosphere but when asked about
it in later years would say: “You know, it applies much better than it should.”
Debye was also a much appreciated lecturer at Cornell University in the ’50’s—particularly when he
illustrated the random nature of diffusion movements by doing his “drunkard’s walk” in front of the class.
However, his eagerness to be an effective administrator was not so clearly manifest and after a year as
Head of the Chemistry Department, he returned back to full-time research and teaching.