Page 363 - MODERN ELECTROCHEMISTRY
P. 363
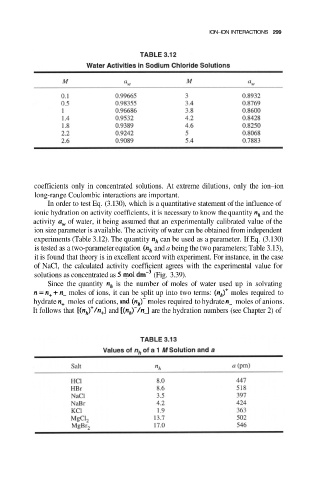
ION–ION INTERACTIONS 299
coefficients only in concentrated solutions. At extreme dilutions, only the ion–ion
long-range Coulombic interactions are important.
In order to test Eq. (3.130), which is a quantitative statement of the influence of
ionic hydration on activity coefficients, it is necessary to know the quantity and the
activity of water, it being assumed that an experimentally calibrated value of the
ion size parameter is available. The activity of water can be obtained from independent
experiments (Table 3.12). The quantity can be used as a parameter. If Eq. (3.130)
is tested as a two-parameter equation and a being the two parameters; Table 3.13),
it is found that theory is in excellent accord with experiment. For instance, in the case
of NaCl, the calculated activity coefficient agrees with the experimental value for
solutions as concentrated as (Fig. 3.39).
Since the quantity is the number of moles of water used up in solvating
moles of ions, it can be split up into two terms: moles required to
hydrate moles of cations, and moles required to hydrate moles of anions.
It follows that and are the hydration numbers (see Chapter 2) of