Page 66 - MODERN ELECTROCHEMISTRY
P. 66
ELECTROCHEMISTRY 11
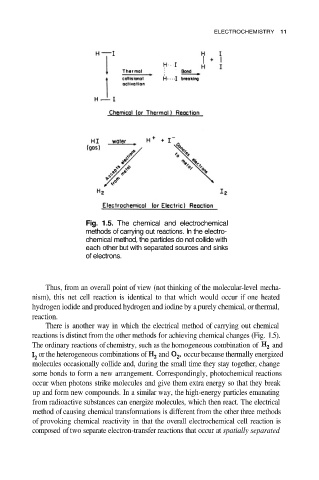
Fig. 1.5. The chemical and electrochemical
methods of carrying out reactions. In the electro-
chemical method, the particles do not collide with
each other but with separated sources and sinks
of electrons.
Thus, from an overall point of view (not thinking of the molecular-level mecha-
nism), this net cell reaction is identical to that which would occur if one heated
hydrogen iodide and produced hydrogen and iodine by a purely chemical, or thermal,
reaction.
There is another way in which the electrical method of carrying out chemical
reactions is distinct from the other methods for achieving chemical changes (Fig. 1.5).
The ordinary reactions of chemistry, such as the homogeneous combination of and
or the heterogeneous combinations of and occur because thermally energized
molecules occasionally collide and, during the small time they stay together, change
some bonds to form a new arrangement. Correspondingly, photochemical reactions
occur when photons strike molecules and give them extra energy so that they break
up and form new compounds. In a similar way, the high-energy particles emanating
from radioactive substances can energize molecules, which then react. The electrical
method of causing chemical transformations is different from the other three methods
of provoking chemical reactivity in that the overall electrochemical cell reaction is
composed of two separate electron-transfer reactions that occur at spatially separated