Page 64 - MODERN ELECTROCHEMISTRY
P. 64
ELECTROCHEMISTRY 9
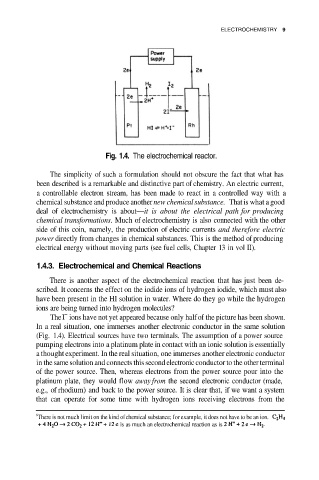
Fig. 1.4. The electrochemical reactor.
The simplicity of such a formulation should not obscure the fact that what has
been described is a remarkable and distinctive part of chemistry. An electric current,
a controllable electron stream, has been made to react in a controlled way with a
chemical substance and produce another new chemical substance. That is what a good
deal of electrochemistry is about—it is about the electrical path for producing
chemical transformations. Much of electrochemistry is also connected with the other
side of this coin, namely, the production of electric currents and therefore electric
power directly from changes in chemical substances. This is the method of producing
electrical energy without moving parts (see fuel cells, Chapter 13 in vol II).
1.4.3. Electrochemical and Chemical Reactions
There is another aspect of the electrochemical reaction that has just been de-
scribed. It concerns the effect on the iodide ions of hydrogen iodide, which must also
have been present in the HI solution in water. Where do they go while the hydrogen
ions are being turned into hydrogen molecules?
The ions have not yet appeared because only half of the picture has been shown.
In a real situation, one immerses another electronic conductor in the same solution
(Fig. 1.4). Electrical sources have two terminals. The assumption of a power source
pumping electrons into a platinum plate in contact with an ionic solution is essentially
a thought experiment. In the real situation, one immerses another electronic conductor
in the same solution and connects this second electronic conductor to the other terminal
of the power source. Then, whereas electrons from the power source pour into the
platinum plate, they would flow away from the second electronic conductor (made,
e.g., of rhodium) and back to the power source. It is clear that, if we want a system
that can operate for some time with hydrogen ions receiving electrons from the
6
There is not much limit on the kind of chemical substance; for example, it does not have to be an ion.
is as much an electrochemical reaction as is