Page 60 - MODERN ELECTROCHEMISTRY
P. 60
ELECTROCHEMISTRY 5
important and growing areas of electrochemistry: bioelectrochemistry and the most
active and expanding of all, the electrochemistry of cleaner environments.
What is the connection between the two main areas in electrochemistry—the
science of solutions (ionics) and that of charge transfer across solid–solution interfaces
(electrodics)? There is indeed a close connection. The interfacial region at electrodes
(and all wet surfaces, including the surface of plants undergoing photosynthesis) is
surrounded by ions in solution (or in the moisture films on surfaces). Thus it is
important that we know all about them. The electrode is the stage; the solution is the
theater and the audience. It is also the place that supplies the players—ions and
solvent—while electrons are clearly supplied from resources in the wings.
1.3. SOME CHARACTERISTICS OF ELECTRODICS
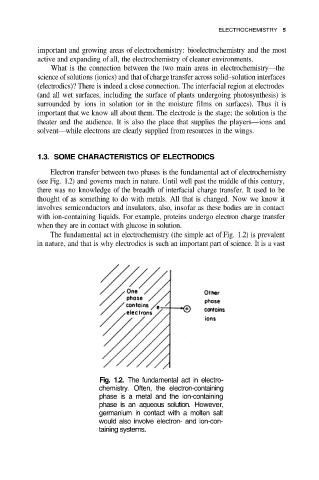
Electron transfer between two phases is the fundamental act of electrochemistry
(see Fig. 1.2) and governs much in nature. Until well past the middle of this century,
there was no knowledge of the breadth of interfacial charge transfer. It used to be
thought of as something to do with metals. All that is changed. Now we know it
involves semiconductors and insulators, also, insofar as these bodies are in contact
with ion-containing liquids. For example, proteins undergo electron charge transfer
when they are in contact with glucose in solution.
The fundamental act in electrochemistry (the simple act of Fig. 1.2) is prevalent
in nature, and that is why electrodics is such an important part of science. It is a vast
Fig. 1.2. The fundamental act in electro-
chemistry. Often, the electron-containing
phase is a metal and the ion-containing
phase is an aqueous solution. However,
germanium in contact with a molten salt
would also involve electron- and ion-con-
taining systems.