Page 62 - MODERN ELECTROCHEMISTRY
P. 62
ELECTROCHEMISTRY 7
electric charges on both sides of the interface. Consider the interior of a metal. It
consists of a lattice of metallic ions populated by electrons in the plasma state that are
mobile and moving randomly at about
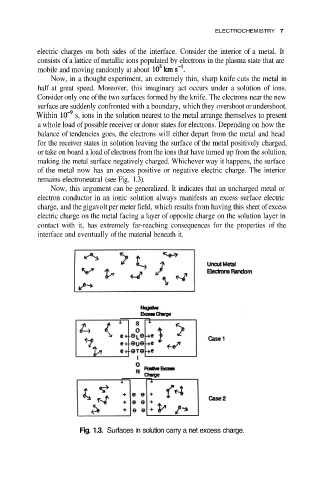
Now, in a thought experiment, an extremely thin, sharp knife cuts the metal in
half at great speed. Moreover, this imaginary act occurs under a solution of ions.
Consider only one of the two surfaces formed by the knife. The electrons near the new
surface are suddenly confronted with a boundary, which they overshoot or undershoot.
Within s, ions in the solution nearest to the metal arrange themselves to present
a whole load of possible receiver or donor states for electrons. Depending on how the
balance of tendencies goes, the electrons will either depart from the metal and head
for the receiver states in solution leaving the surface of the metal positively charged,
or take on board a load of electrons from the ions that have turned up from the solution,
making the metal surface negatively charged. Whichever way it happens, the surface
of the metal now has an excess positive or negative electric charge. The interior
remains electroneutral (see Fig. 1.3).
Now, this argument can be generalized. It indicates that an uncharged metal or
electron conductor in an ionic solution always manifests an excess surface electric
charge, and the gigavolt per meter field, which results from having this sheet of excess
electric charge on the metal facing a layer of opposite charge on the solution layer in
contact with it, has extremely far-reaching consequences for the properties of the
interface and eventually of the material beneath it.
Fig. 1.3. Surfaces in solution carry a net excess charge.