Page 73 - MODERN ELECTROCHEMISTRY
P. 73
ELECTROCHEMISTRY 17
If one goes to a nonaqueous solvent system (an organic one such as acetonitrile,
or a pure electrolyte such as the eutectic), the dielectric
constant is more in the range of 2–20, and there is a greater tendency than that in
aqueous solutions for ions of opposite sign to get together and stay together. Further,
the bonding that develops is not purely Coulombic but may involve solvent and H-
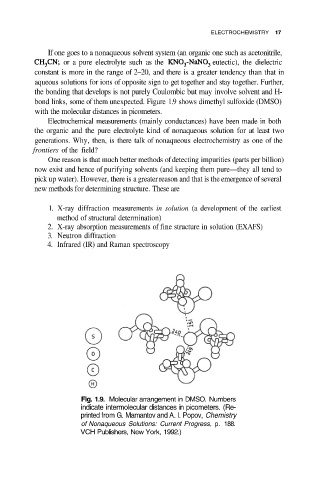
bond links, some of them unexpected. Figure 1.9 shows dimethyl sulfoxide (DMSO)
with the molecular distances in picometers.
Electrochemical measurements (mainly conductances) have been made in both
the organic and the pure electrolyte kind of nonaqueous solution for at least two
generations. Why, then, is there talk of nonaqueous electrochemistry as one of the
frontiers of the field?
One reason is that much better methods of detecting impurities (parts per billion)
now exist and hence of purifying solvents (and keeping them pure—they all tend to
pick up water). However, there is a greater reason and that is the emergence of several
new methods for determining structure. These are
1. X-ray diffraction measurements in solution (a development of the earliest
method of structural determination)
2. X-ray absorption measurements of fine structure in solution (EXAFS)
3. Neutron diffraction
4. Infrared (IR) and Raman spectroscopy
Fig. 1.9. Molecular arrangement in DMSO. Numbers
indicate intermolecular distances in picometers. (Re-
printed from G. Mamantov and A. I. Popov, Chemistry
of Nonaqueous Solutions: Current Progress, p. 188.
VCH Publishers, New York, 1992.)