Page 75 - MODERN ELECTROCHEMISTRY
P. 75
ELECTROCHEMISTRY 19
1.7. A NEW WORLD OF RICH VARIETY: ROOM-TEMPERATURE
MOLTEN SALTS
The image in most chemists’ minds of a “molten salt” is probably liquid NaCl at
900 °C. There are many such “pure electrolytes” that have the great advantage of a
large electrochemical “window”; that is, one can carry out electrode processes in them
over a much greater range of cell potentials than is possible in aqueous solutions, where
the range is limited by the evolution of if the potential becomes too negative and
of O 2 if it becomes too positive.
This situation has been radically altered during the past 20 years or so by work
that has been led (in separate and individual ways) by three U.S. electrochemists,
namely, Osteryoung, Hussey, and Wilkes, respectively. Thus, the first truly room-
temperature pure electrolyte (i.e., a system consisting of ions without a solvent) was
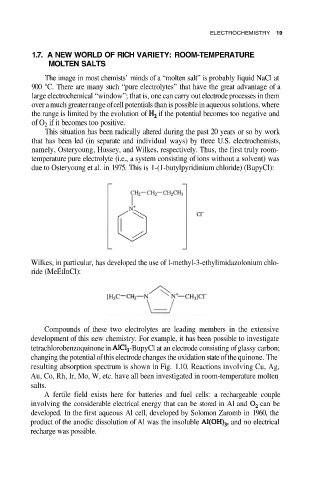
due to Osteryoung et al. in 1975. This is 1-(1-butylpyridinium chloride) (BupyCl):
Wilkes, in particular, has developed the use of l-methyl-3-ethylimidazolonium chlo-
ride (MeEtInCl):
Compounds of these two electrolytes are leading members in the extensive
development of this new chemistry. For example, it has been possible to investigate
tetrachlorobenzoquinone in -BupyCl at an electrode consisting of glassy carbon;
changing the potential of this electrode changes the oxidation state of the quinone. The
resulting absorption spectrum is shown in Fig. 1.10. Reactions involving Cu, Ag,
Au, Co, Rh, Ir, Mo, W, etc. have all been investigated in room-temperature molten
salts.
A fertile field exists here for batteries and fuel cells: a rechargeable couple
involving the considerable electrical energy that can be stored in Al and can be
developed. In the first aqueous Al cell, developed by Solomon Zaromb in 1960, the
product of the anodic dissolution of Al was the insoluble and no electrical
recharge was possible.