Page 79 - MODERN ELECTROCHEMISTRY
P. 79
ELECTROCHEMISTRY 23
the enzyme because eventually the electrons from the reaction on its surface (in contact
with the blood) must get through the 10 nm of enzyme to the metallic circuitry of the
wrist meter.
Now, most enzymes are centered on a specific redox atom (e.g., Fe) and in order
to be oxidized or reduced, the electron, the effector of the act, must travel through the
enzyme to the so-called heme group, the vital Fe-containing group, as in hemoglobin,
for example.
Enzymes are complex organic substances and are not expected to be good electron
conductors at all. If an electron is going to get to and from the heme group to an outside
contact, the best hope is quantum mechanical tunneling. However, there is a limit to
the jump length in tunneling; it is about 2 nm. Supposing the heme group is in the
middle of the enzyme glucose oxidase; then, as it is ~9 nm across, the electron would
have to jump ~4 nm, which is not possible.
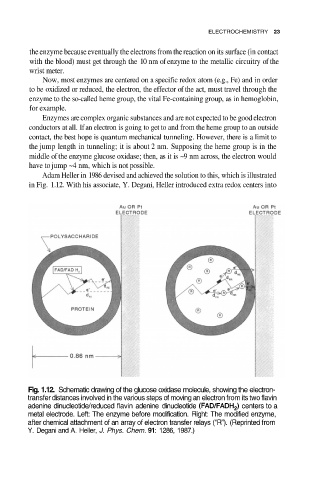
Adam Heller in 1986 devised and achieved the solution to this, which is illustrated
in Fig. 1.12. With his associate, Y. Degani, Heller introduced extra redox centers into
Fig. 1.12. Schematic drawing of the glucose oxidase molecule, showing the electron-
transfer distances involved in the various steps of moving an electron from its two flavin
adenine dinucleotide/reduced flavin adenine dinucleotide centers to a
metal electrode. Left: The enzyme before modification. Right: The modified enzyme,
after chemical attachment of an array of electron transfer relays (“R”). (Reprinted from
Y. Degani and A. Heller, J. Phys. Chem. 91: 1286, 1987.)