Page 82 - MODERN ELECTROCHEMISTRY
P. 82
26 CHAPTER 1
1. Concentration and temperature determine the rate at which chemical proc-
esses take place. Electrochemical processes are equally affected by these variables,
but also are controlled in selectivity of reaction by the electrical potential of the
electrode (see Section XXX). Hence, there is an extra variable that controls chemical
processes that occur electrochemically rather than chemically. Moreover, this variable
is applied easily by turning the knob on an electrical power source.
2. When electrochemical processes are used to clean up carbonaceous material,
the only gas produced is there are no noxious products of partial combustion,
such as NO and CO, to be injected into the atmosphere. When hydrogen is used in a
fuel cell to produce electric power, it is made by splitting water and it produces water
right back again as a by-product of the power generation.
This book contains several examples of electrochemical clean-up processes (see
Chapter 15), but one is briefly described here. It is in the cleanup of wastewater, defined
as water having impurities in the range of 5–500 ppm. There is a problem in using
an electrochemical approach because of the low electrical conductance of the
water. However, in one of the several electrochemical companies developed
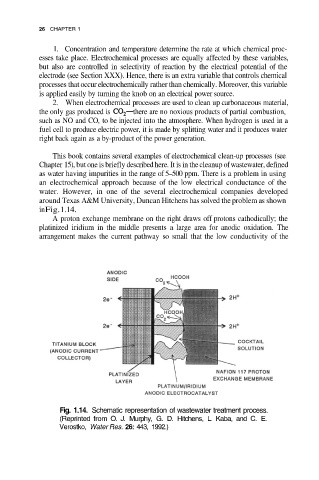
around Texas A&M University, Duncan Hitchens has solved the problem as shown
in Fig. 1.14.
A proton exchange membrane on the right draws off protons cathodically; the
platinized iridium in the middle presents a large area for anodic oxidation. The
arrangement makes the current pathway so small that the low conductivity of the
Fig. 1.14. Schematic representation of wastewater treatment process.
(Reprinted from O. J. Murphy, G. D. Hitchens, L. Kaba, and C. E.
Verostko, Water Res. 26: 443, 1992.)