Page 92 - MODERN ELECTROCHEMISTRY
P. 92
CHAPTER 2
ION–SOLVENT INTERACTIONS
2.1. INTRODUCTION
Aristotle noted that one could separate water from a solution by means of
evaporation. Some two millennia later, Fourcroy in 1800 focused attention on the
interaction of a solute with its solvent.
These early observations serve to introduce a subject—the formation of mobile
ions in solution—that is as basic to electrochemistry as is the process often considered
its fundamental act: the transfer of an electron across the double layer to or from an
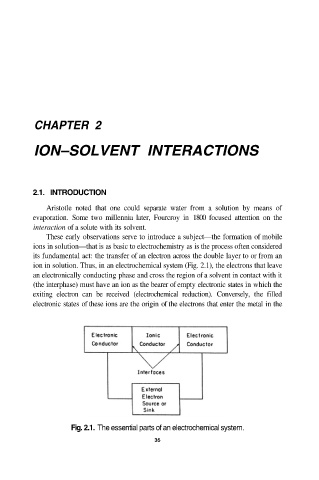
ion in solution. Thus, in an electrochemical system (Fig. 2.1), the electrons that leave
an electronically conducting phase and cross the region of a solvent in contact with it
(the interphase) must have an ion as the bearer of empty electronic states in which the
exiting electron can be received (electrochemical reduction). Conversely, the filled
electronic states of these ions are the origin of the electrons that enter the metal in the
Fig. 2.1. The essential parts of an electrochemical system.
35