Page 180 - Modern physical chemistry
P. 180
172 Equilibria in Condensed Phases
Each of these procedures involves a different k i in equation (7.42), a different pOi in
formula (8.2), and so a different standard state. But for anyone of these choices, a person
can construct equation (7.52) for AG, set it equal to zero as in equation (7.54), and obtain
the conventional form for the equilibrium constant (7.57).
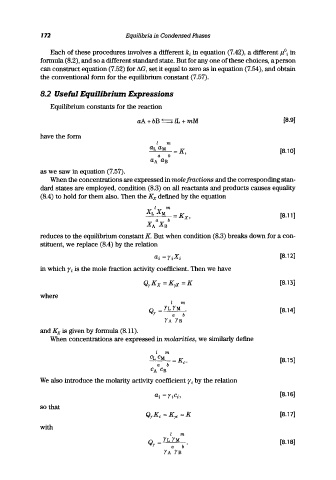
8.2 Useful Equilibrium Expressions
Equilibrium constants for the reaction
aA + bB ( ) lL + mM [8.9J
have the form
[8.lOJ
as we saw in equation (7.57).
When the concentrations are expressed in mole fractions and the corresponding stan-
dard states are employed, condition (8.3) on all reactants and products causes equality
(8.4) to hold for them also. Then the Kx defined by the equation
I m
XL X M _ K [8.11 J
a b - X,
X A X B
reduces to the equilibrium constant K. But when condition (8.3) breaks down for a con-
stituent, we replace (8.4) by the relation
ai =YiXi [8.12J
in which ri is the mole fraction activity coefficient. Then we have
[8.13J
where
I m
Q = YL YM [8.14J
Y a b
YA YB
and Kx is given by formula (8.11).
When concentrations are expressed in molarities, we similarly define
I m
CL CM -K [8.15J
a b - c·
CA CB
We also introduce the molarity activity coefficient ri by the relation
[8.16J
so that
[8.17J
with
I m
Q = YL YM [8.18J
Y a b'
YA YB