Page 182 - Modern physical chemistry
P. 182
174 Equilibria in Condensed Phases
we have
[8.31 J
But note that writing (8.30) in the form
H 20 (1) ( ) H+ (aq) + OH- (aq) [8.32J
yields
_ aH+a OH - _
K - - aH+a OH -' [8.33J
aH20
essentially the same final form. For other reactions, a similar remark applies. So in equi-
librium studies, we will symbolize the hydrogen ion in water merely as W (aq).
When a crystal is effectively pure and under 1 bar pressure, it is in its standard state
and its actiVity may be taken as 1. For the reaction
[8.34J
we have
[8.35J
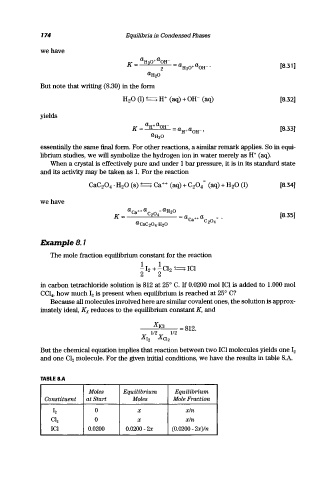
ExampleB.l
The mole fraction equilibrium constant for the reaction
1 1
- 12 + -C1 2 ( ) ICI
2 2
in carbon tetrachloride solution is 812 at 25° C. If 0.0200 mol ICI is added to 1.000 mol
CCI 4 , how much 12 is present when equilibrium is reached at 25° C?
Because all molecules involved here are similar covalent ones, the solution is approx-
imately ideal, Kx reduces to the equilibrium constant K, and
__ X-,-IC=I __ = 812.
112 112
X I2 X CI2
But the chemical equation implies that reaction between two ICI molecules yields one 12
and one Cl 2 molecule. For the given initial conditions, we have the results in table 8.A.
TABLE S.A
Moles Equilibrium Equilibrium
Constituent at Start Moles Mole Fraction
12 0 x x/n
CI 2 0 x x/n
ICI 0.0200 0.0200-2x (0.0200 - 2x)/n