Page 185 - Modern physical chemistry
P. 185
B.3 Conditions Determining Equilibria 171
concentrations are related to the initial ones through the coefficients in the chemical
equations. The final concentrations are related to each other by the equilibrium expres-
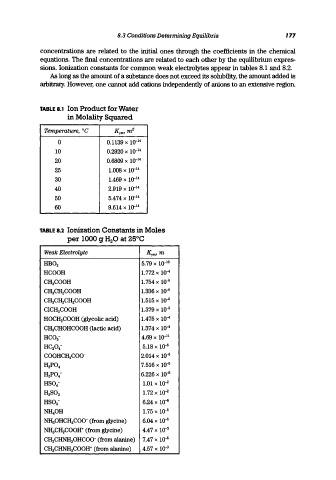
sions. Ionization constants for common weak electrolytes appear in tables 8.1 and 8.2.
As long as the amount of a substance does not exceed its solubility, the amount added is
arbitrary. However, one cannot add cations independently of anions to an extensive region.
TABLE 8.1 Ion Product for Water
in Molality Squared
Temperature, °C Kym, m 2
0 0.1139 x 10- 14
10 0.2920 X 10- 14
20 0.6809 X 10- 14
25 1.008 x 10- 14
30 1.469 X 10- 14
40 2.919 X 10- 14
50 5.474 X 10- 14
60 9.614 X 10- 14
TABLE 8.2 Ionization Constants in Moles
per 1000 9 H 20 at 25°C
Weak Electrolyte Kym,m
HB0 2 5.79 x 10- 10
HCOOH 1.772 x 10-4
CH 3COOH 1.754 x 10-0
CH 3CH 2COOH 1.336 x 10-0
CH 3CH 2CH 2COOH 1.515 x 10-0
CICH 2COOH 1.379 x 10-3
HOCH 2COOH (glycolic acid) 1.475 x 1Q-4
CH 3CHOHCOOH (lactic acid) 1.374 x 10-4
HC0 3 - 4.69 X 10- 11
HC 20 4 - 5.18 x 10-0
COOHCH 2COO- 2.014 x 10..0
7.516 x 10-3
H 3P0 4
H 2 P0 4 - 6.226 x 10-0
HS0 4 - 1.01 X 10- 2
1.72 X 10-2
H 2S0 3
HS0 3 - 6.24 x 10-0
NH 40H 1.75 x 10-0
NH 30HCH 2COO- (from glycine) 6.04 x 10-0
NH 3CH 2COOH+ (from glycine) 4.47 x 1o-a
CH 3CHNH 30HCOO- (from alanine) 7.47 x 10-0
CH 3CHNHa COOH+ (from alanine) 4.57 x 1o-a