Page 21 - Modern physical chemistry
P. 21
10 Structure in Solids
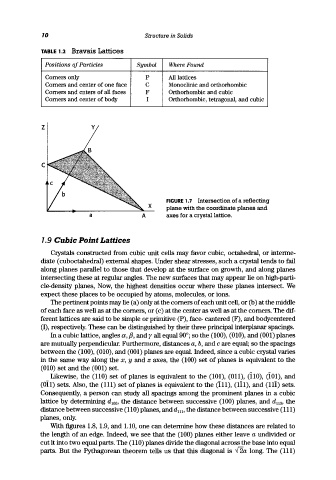
TABLE 1.2 Bravais Lattices
Positions of Particles Symbol Where Found
Corners only P All lattices
Corners and center of one face C Monoclinic and orthorhombic
Corners and cnters of all faces F Orthorhombic and cubic
Corners and center of body I Orthorhombic, tetragonal, and cubic
z y
FIGURE 1.7 Intersection of a reflecting
x plane with the coordinate planes and
a A axes for a crystal lattice.
1.9 Cubic Point Lattices
Crystals constructed from cubic unit cells may favor cubic, octahedral, or interme-
diate (cuboctahedral) external shapes. Under shear stresses, such a crystal tends to fail
along planes parallel to those that develop at the surface on growth, and along planes
intersecting these at regular angles. The new surfaces that may appear lie on high-parti-
de-density planes, Now, the highest densities occur where these planes intersect. We
expect these places to be occupied by atoms, molecules, or ions.
The pertinent points may lie ( a) only at the comers of each unit cell, or (b) at the middle
of each face as well as at the comers, or (c) at the center as well as at the comers. The dif-
ferent lattices are said to be simple or primitive (P), face- cantered (F), and bodycentered
(1), respectively. These can be distinguished by their three principal interplanar spacings.
In a cubic lattice, angles a, (J, and r all equal 90°; so the (100), (010), and (001) planes
are mutually perpendicular. Furthermore, distances a, b, and c are equal; so the spacings
between the (100), (010), and (001) planes are equal. Indeed, since a cubic crystal varies
in the same way along the x, y and z axes, the (100) set of planes is equivalent to the
(010) set and the (001) set.
Likewise, the (110) set of planes is equivalent to the (101), (011), (ilO), (101), and
(011) sets. Also, the (111) set of planes is equivalent to the (111), (lil), and (111) sets.
Consequently, a person can study all spacings among the prominent planes in a cubic
lattice by determining d lOO , the distance between successive (100) planes, and duo, the
distance between successive (110) planes, and d lll , the distance between successive (111)
planes, only.
With figures 1.8, 1.9, and 1.10, one can determine how these distances are related to
the length of an edge. Indeed, we see that the (100) planes either leave a undivided or
cut it into two equal parts. The (110) planes divide the diagonal across the base into equal
parts. But the Pythagorean theorem tells us that this diagonal is ...f2a long. The (111)