Page 25 - Modern physical chemistry
P. 25
14 Structure in Solids
~---;1---~
If' II II
I I
I I
}f-------}!f !.-_I~ -J I I I
I
II I~~/I--'
I I I I I I I I'I f" I I I I
I I I I
I I I I '" ~ 1;- -r~LA I
~ ~-~ OW-~
~--L----~ I
T I I I I II /i,,/Jr-/.I I I
I I 0 I I I I ! ,(./J:/ I I I I I
: ~----t-~ r-;'01=F" -1- -/'
I I
I I
I I
II I I t---%I __ ~
I I I I
I I
I I I I
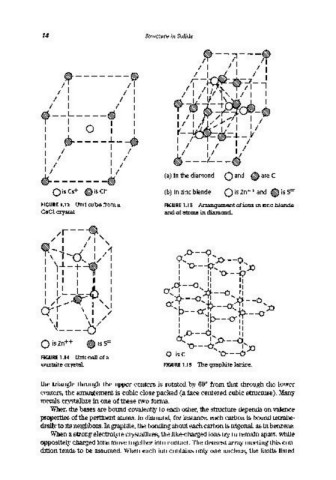
~------~ (a) In the diamond 0 and ~ are (
Ois (s+ ~is (1- (b) In zinc blende o is Zn++ and ~ is S=
FIGURE 1.12 Unit cube from a FIGURE 1.13 Arrangement of ions in zinc blende
CsCl crystal and of atoms in diamond.
/r--~
/ I I I',
~_..! I I "" .!)---o
~\ I-d I / I (1.... 1'.0---0
I -+
I \.l '~/ I ,o---q.... I ':n
I I
I I I I II "O---Q
I I I I II I I II II
I I ,.0..--0 II II
I I !..._L~ I 0 .... I '*.--0 II
....
I /( -~- I ' : cr*--o .... .k ~--O
;0
.... 1-
____ +r--o .... ~
qr' \ I '" t·· r - -v. ..J -t(" - -0'
\ I \ I / I IP---o'il
~----O/ I II II
I .!)---O
II
0.... 1';:>---0
o isZn++ ~ isS= '0- - -0.... I ':n
o is ( '0---0
FIGURE 1.14 Unit cell of a
wurtzite crystal. FIGURE 1.15 The graphite lattice.
the triangle through the upper centers is rotated by 60° from that through the lower
centers, the arrangement is cubic close packed (a face centered cubic structure). Many
metals crystallize in one of these two forms.
When the bases are bound covalently to each other, the structure depends on valence
properties of the pertinent atoms. In diamond, for instance, each carbon is bound tetrahe-
drally to its neighbors. In graphite, the bonding about each carbon is trigonal, as in benzene.
When a strong electrolyte crystallizes, the like-charged ions try to remain apart, while
oppositely charged ions move together into contact. The densest array meeting this con-
dition tends to be assumed. When each ion contains only one nucleus, the limits listed