Page 189 - Multidimensional Chromatography
P. 189
182 Multidimensional Chromatography
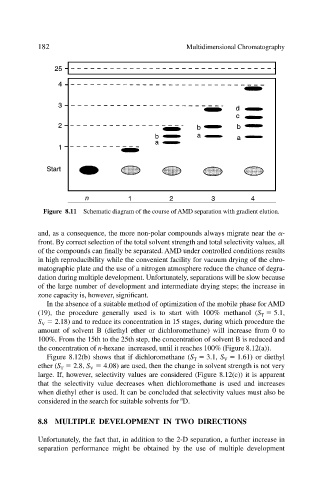
Figure 8.11 Schematic diagram of the course of AMD separation with gradient elution.
and, as a consequence, the more non-polar compounds always migrate near the -
front. By correct selection of the total solvent strength and total selectivity values, all
of the compounds can finally be separated. AMD under controlled conditions results
in high reproducibility while the convenient facility for vacuum drying of the chro-
matographic plate and the use of a nitrogen atmosphere reduce the chance of degra-
dation during multiple development. Unfortunately, separations will be slow because
of the large number of development and intermediate drying steps; the increase in
zone capacity is, however, significant.
In the absence of a suitable method of optimization of the mobile phase for AMD
(19), the procedure generally used is to start with 100% methanol (S T 5.1,
S V 2.18) and to reduce its concentration in 15 stages, during which procedure the
amount of solvent B (diethyl ether or dichloromethane) will increase from 0 to
100%. From the 15th to the 25th step, the concentration of solvent B is reduced and
the concentration of n-hexane increased, until it reaches 100% (Figure 8.12(a)).
Figure 8.12(b) shows that if dichloromethane (S T 3.1, S V 1.61) or diethyl
ether (S T 2.8, S V 4.08) are used, then the change in solvent strength is not very
large. If, however, selectivity values are considered (Figure 8.12(c)) it is apparent
that the selectivity value decreases when dichloromethane is used and increases
when diethyl ether is used. It can be concluded that selectivity values must also be
n
considered in the search for suitable solvents for D.
8.8 MULTIPLE DEVELOPMENT IN TWO DIRECTIONS
Unfortunately, the fact that, in addition to the 2-D separation, a further increase in
separation performance might be obtained by the use of multiple development