Page 205 - Multidimensional Chromatography
P. 205
198 Multidimensional Chromatography
Many of the first electrophoretic separations were conducted on planar media.
Convection due to Joule heating was reduced in solid support materials, such as cel-
lulose filter paper and polyacrylamide gels, as compared to free solution separations.
An open tubular format was desired, but limitations in available materials and tubing
diameters presented Joule heating issues. As a result, capillary columns were first
employed for electrodriven separation in 1979 (3). In 1981, Jorgenson and De
Arman Lukacs introduced free solution electrophoresis in 75 m glass capillaries, a
technique that was named capillary zone electrophoresis (CZE) (4). The main
benefits of CZE were the capillary format that reduced Joule heating effects and the
free solution separation, which eliminated eddy diffusion as a contributor to zone
spreading.
Capillary electrophoresis (CE) today is available in many diversified forms, such
as capillary isotachaphoresis and capillary electrochromatography. It is a technique
offering very high resolution and efficiency. CE can separate both ionic and non-
ionic compounds over an exceedingly broad range of molecular weights. The on-line
detection of CE usually yields good quantitative results, but poor mass sensitivity
due to the small volumes of sample used. The capillary format in CE yields other
benefits aside from the efficient dissipation of heat, including the requirement of
only small amounts of sample and minimal solvent usage.
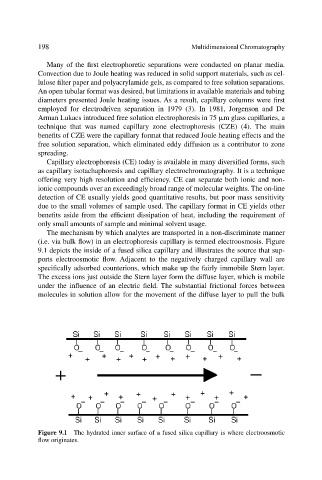
The mechanism by which analytes are transported in a non-discriminate manner
(i.e. via bulk flow) in an electrophoresis capillary is termed electroosmosis. Figure
9.1 depicts the inside of a fused silica capillary and illustrates the source that sup-
ports electroosmotic flow. Adjacent to the negatively charged capillary wall are
specifically adsorbed counterions, which make up the fairly immobile Stern layer.
The excess ions just outside the Stern layer form the diffuse layer, which is mobile
under the influence of an electric field. The substantial frictional forces between
molecules in solution allow for the movement of the diffuse layer to pull the bulk
Figure 9.1 The hydrated inner surface of a fused silica capillary is where electroosmotic
flow originates.