Page 319 - Multidimensional Chromatography
P. 319
Industrial and Polymer Applications 309
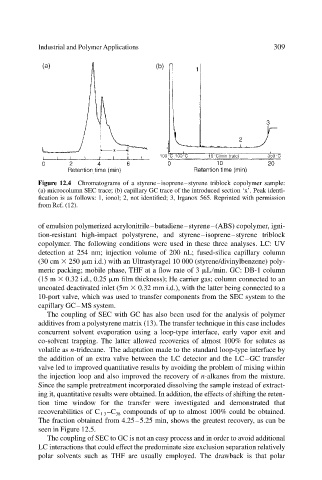
Figure 12.4 Chromatograms of a styrene–isoprene–styrene triblock copolymer sample:
(a) microcolumn SEC trace; (b) capillary GC trace of the introduced section ‘x’. Peak identi-
fication is as follows: 1, ionol; 2, not identified; 3, Irganox 565. Reprinted with permission
from Ref. (12).
of emulsion polymerized acrylonitrile–butadiene–styrene–(ABS) copolymer, igni-
tion-resistant high-impact polystyrene, and styrene–isoprene–styrene triblock
copolymer. The following conditions were used in these three analyses. LC: UV
detection at 254 nm; injection volume of 200 nL; fused-silica capillary column
(30 cm 250 m i.d.) with an Ultrastyragel 10 000 (styrene/divinylbenzene) poly-
meric packing; mobile phase, THF at a flow rate of 3 L min. GC: DB-1 column
(15 m 0.32 i.d., 0.25 m film thickness); He carrier gas; column connected to an
uncoated deactivated inlet (5m 0.32 mm i.d.), with the latter being connected to a
10-port valve, which was used to transfer components from the SEC system to the
capillary GC–MS system.
The coupling of SEC with GC has also been used for the analysis of polymer
additives from a polystyrene matrix (13). The transfer technique in this case includes
concurrent solvent evaporation using a loop-type interface, early vapor exit and
co-solvent trapping. The latter allowed recoveries of almost 100% for solutes as
volatile as n-tridecane. The adaptation made to the standard loop-type interface by
the addition of an extra valve between the LC detector and the LC–GC transfer
valve led to improved quantitative results by avoiding the problem of mixing within
the injection loop and also improved the recovery of n-alkanes from the mixture.
Since the sample pretreatment incorporated dissolving the sample instead of extract-
ing it, quantitative results were obtained. In addition, the effects of shifting the reten-
tion time window for the transfer were investigated and demonstrated that
recoverabilities of C 13 –C 38 compounds of up to almost 100% could be obtained.
The fraction obtained from 4.25–5.25 min, shows the greatest recovery, as can be
seen in Figure 12.5.
The coupling of SEC to GC is not an easy process and in order to avoid additional
LC interactions that could effect the predominate size exclusion separation relatively
polar solvents such as THF are usually employed. The drawback is that polar