Page 326 - Multidimensional Chromatography
P. 326
316 Multidimensional Chromatography
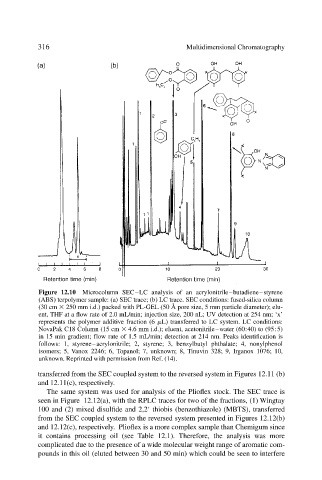
Figure 12.10 Microcolumn SEC–LC analysis of an acrylonitrile–butadiene–styrene
(ABS) terpolymer sample: (a) SEC trace; (b) LC trace. SEC conditions: fused-silica column
(30 cm 250 mm i.d.) packed with PL-GEL (50 Å pore size, 5 mm particle diameter); elu-
ent, THF at a flow rate of 2.0 mL/min; injection size, 200 nL; UV detection at 254 nm; ‘x’
represents the polymer additive fraction (6 L) transferred to LC system. LC conditions:
NovaPak C18 Column (15 cm 4.6 mm i.d.); eluent, acetonitrile–water (60:40) to (95:5)
in 15 min gradient; flow rate of 1.5 mL/min; detection at 214 nm. Peaks identification is
follows: 1, styrene–acrylonitrile; 2, styrene; 3, benzylbutyl phthalate; 4, nonylphenol
isomers; 5, Vanox 2246; 6, Topanol; 7, unknown; 8, Tinuvin 328; 9, Irganox 1076; 10,
unknown. Reprinted with permission from Ref. (14).
transferred from the SEC coupled system to the reversed system in Figures 12.11 (b)
and 12.11(c), respectively.
The same system was used for analysis of the Plioflex stock. The SEC trace is
seen in Figure 12.12(a), with the RPLC traces for two of the fractions, (1) Wingtay
100 and (2) mixed disulfide and 2,2 thiobis (benzothiazole) (MBTS), transferred
from the SEC coupled system to the reversed system presented in Figures 12.12(b)
and 12.12(c), respectively. Plioflex is a more complex sample than Chemigum since
it contains processing oil (see Table 12.1). Therefore, the analysis was more
complicated due to the presence of a wide molecular weight range of aromatic com-
pounds in this oil (eluted between 30 and 50 min) which could be seen to interfere