Page 46 - Multidimensional Chromatography
P. 46
36 Multidimensional Chromatography
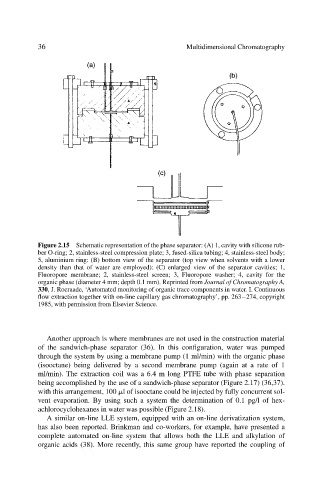
Figure 2.15 Schematic representation of the phase separator: (A) 1, cavity with silicone rub-
ber O-ring; 2, stainless-steel compression plate; 3, fused-silica tubing; 4, stainless-steel body;
5, aluminium ring: (B) bottom view of the separator (top view when solvents with a lower
density than that of water are employed): (C) enlarged view of the separator cavities; 1,
Fluoropore membrane; 2, stainless-steel screen; 3, Fluoropore washer; 4, cavity for the
organic phase (diameter 4 mm; depth 0.1 mm). Reprinted from Journal of Chromatography A,
330, J. Roeraade, ‘Automated monitoring of organic trace components in water. I. Continuous
flow extraction together with on-line capillary gas chromatography’, pp. 263–274, copyright
1985, with permission from Elsevier Science.
Another approach is where membranes are not used in the construction material
of the sandwich-phase separator (36). In this configuration, water was pumped
through the system by using a membrane pump (1 ml/min) with the organic phase
(isooctane) being delivered by a second membrane pump (again at a rate of 1
ml/min). The extraction coil was a 6.4 m long PTFE tube with phase separation
being accomplished by the use of a sandwich-phase separator (Figure 2.17) (36,37).
with this arrangement, 100 l of isooctane could be injected by fully concurrent sol-
vent evaporation. By using such a system the determination of 0.1 pg/l of hex-
achlorocyclohexanes in water was possible (Figure 2.18).
A similar on-line LLE system, equipped with an on-line derivatization system,
has also been reported. Brinkman and co-workers, for example, have presented a
complete automated on-line system that allows both the LLE and alkylation of
organic acids (38). More recently, this same group have reported the coupling of