Page 48 - Multidimensional Chromatography
P. 48
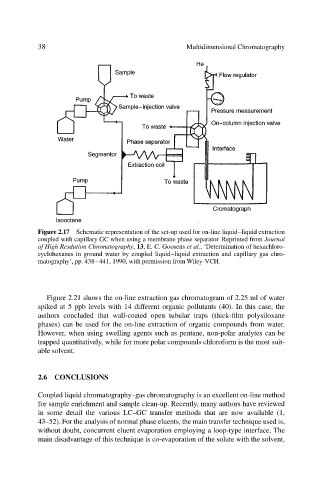
38 Multidimensional Chromatography
Figure 2.17 Schematic representation of the set-up used for on-line liquid–liquid extraction
coupled with capillary GC when using a membrane phase separator. Reprinted from Journal
of High Resdution Chromatography, 13, E. C. Goosens et al., ‘Determination of hexachloro-
cyclohexanes in ground water by coupled liquid–liquid extraction and capillary gas chro-
matography’, pp. 438–441, 1990, with permission from Wiley-VCH.
Figure 2.21 shows the on-line extraction gas chromatogram of 2.25 ml of water
spiked at 5 ppb levels with 14 different organic pollutants (40). In this case, the
authors concluded that wall-coated open tubular traps (thick-film polysiloxane
phases) can be used for the on-line extraction of organic compounds from water.
However, when using swelling agents such as pentane, non-polar analytes can be
trapped quantitatively, while for more polar compounds chloroform is the most suit-
able solvent.
2.6 CONCLUSIONS
Coupled liquid chromatography–gas chromatography is an excellent on-line method
for sample enrichment and sample clean-up. Recently, many authors have reviewed
in some detail the various LC–GC transfer methods that are now available (1,
43–52). For the analysis of normal phase eluents, the main transfer technique used is,
without doubt, concurrent eluent evaporation employing a loop-type interface. The
main disadvantage of this technique is co-evaporation of the solute with the solvent,