Page 115 - Multifunctional Photocatalytic Materials for Energy
P. 115
104 Multifunctional Photocatalytic Materials for Energy
photocatalyst with a visible light response, high performance, and low cost have not
yet been achieved.
Graphitic carbon nitride, as a metal-free and conjugated polymeric semiconductor
material, has a number of advantages, including high corrosion resistance, good sta-
bility, and an easily controllable structure. Its narrow band gap (2.7 eV) also enables
it to respond to visible light [12]. More strikingly, both the HOMO and the LUMO
encompass the oxidation and reduction potentials of water. This characteristic ensures
–
that the hole in the valence band (VB) is sufficiently reactive to oxidize OH into O 2 ,
and at the same time, the electron in the conduction band (CB) has the real potential
+
to reduce H to hydrogen. The salient features discovered here seem to be in close
proximity to the ideal photocatalyst previously mentioned. Thus, upon the pioneering
report in which Wang et al. [15] used g-C 3 N 4 for water splitting, it instantly became
a hot topic in the field of photocatalysis. Since that time, an ever-increasing number
of papers reporting on the use of carbon nitride to directly convert solar energy into
hydrogen energy have been published [2]. Also, the versatile photocatalyst also rep-
resents an attractive strategy for generation of other renewable energies, for example,
hydrocarbon fuels [16]. Although this conjugated polymer shows a great potential for
solar energy utilization, pristine carbon nitride still suffers from low photocatalytic
efficiency because of its small surface area with limited active sites, its high charge
recombination rate, and its weak ability to harvest visible light. As a result, a myr-
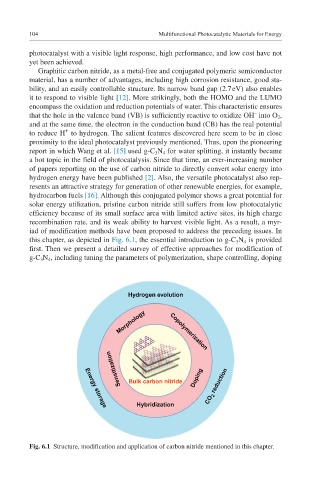
iad of modification methods have been proposed to address the preceding issues. In
this chapter, as depicted in Fig. 6.1, the essential introduction to g-C 3 N 4 is provided
first. Then we present a detailed survey of effective approaches for modification of
g-C 3 N 4 , including tuning the parameters of polymerization, shape controlling, doping
Hydrogen evolution
Morphology Copolymerization
Sensitization Bulk carbon nitride Doping CO 2 reduction
Hybridization
Energy storage
Fig. 6.1 Structure, modification and application of carbon nitride mentioned in this chapter.