Page 119 - Multifunctional Photocatalytic Materials for Energy
P. 119
108
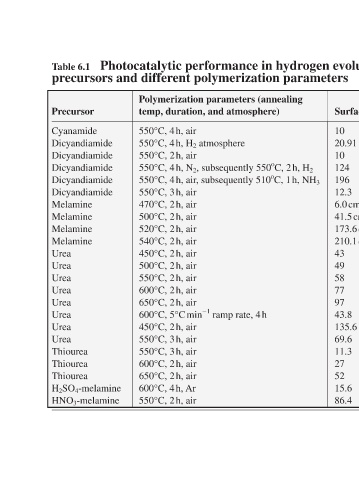
Photocatalytic performance in hydrogen evolution of carbon nitride derived from different
[24]
[22]
[18]
[18]
[18]
[18]
[18]
[18]
[21]
[28]
[25]
[23]
[23]
[18]
19,412 μmol h −1 g −1
417 μmol h −1 g −1
109.1 μmol h −1
151.1 μmol h −1
157.2 μmol h −1
47.2 μmol h −1
89.8 μmol h −1
3.79 μmol h −1
15.1 μmol h −1
23.5 μmol h −1
79.0 μmol h −1
1.4 μmol h −1
3.4 μmol h −1
66 μmol h −1
210.1 cm 2 g −1
135.6
69.6
86.4
15.6
11.3
43.8
precursors and different polymerization parameters
97
43
52
27
58
77
49
Polymerization parameters (annealing Photocatalytic performance in Ref. hydrogen evolution (TEOA, Pt) Surface area/m 2 g −1 temp, duration, and atmosphere) [15] 770 μmol H 2 after 72 h 10 550°C, 4 h, air [20] 4.8 times higher 20.91 550°C, 4 h, H 2 atmosphere [18] 12.1 μmol h −1 10 550°C, 2 h, air [26] 4.8 μmol
600°C, 5°C min −1 ramp rate, 4 h
550°C, 2 h, air
650°C, 2 h, air
650°C, 2 h, air
500°C, 2 h, air
600°C, 2 h, air
450°C, 2 h, air
540°C, 2 h, air
550°C, 3 h, air
550°C, 3 h, air
450°C, 2 h, air
600°C, 2 h, air
550°C, 2 h, air
600°C, 4 h, Ar
Table 6.1 Precursor Cyanamide Dicyandiamide Dicyandiamide Dicyandiamide Dicyandiamide Dicyandiamide Melamine Melamine Melamine Melamine Urea Urea Urea Urea Urea Urea Urea Urea Thiourea Thiourea Thiourea H 2 SO 4 -melamine HNO 3 -melamine