Page 124 - Multifunctional Photocatalytic Materials for Energy
P. 124
Carbon nitride photocatalysts 111
splitting ability of carbon nitride. In consideration of less organic molecules were
reported for copolymerization with the precursors of g-C 3 N 4 , more and more relevant
studies are therefore urged.
6.2.3 Nanostructured carbon nitride
Fabrication of nanostructured materials—such as a porous structure [33]; a 2D
nanosheet [34]; a 1D, that is, a nanotube [35], a nanofiber [36], etc.; and an 0D, that is,
a quantum dot [37] or a hollow nanosphere [38]—is a very effective approach widely
used in the modification of semiconductor materials. The fabrication of nanostruc-
tures for g-C 3 N 4 has demonstrated the capacity to enhance visible light absorption,
enlarge the surface area, and facilitate the electron-hole separation rate in different
morphologies.
In order to increase the BET areas, mesoporous graphitic carbon nitride and
ompg-carbon nitride were prepared by introducing soft or hard templates into the
synthesis. Wang et al. [39] introduced 12 nm SiO 2 particles as a hard template and
cyanamide as the precursor to prepare mesoporous carbon nitride. After removing
the template, mpg-C 3 N 4 was obtained. The surface area of mpg-C 3 N 4 could reach as
2 −1
high as 373 m g , which was dependent on the proportion of precursor to template.
As a result, the rate of H 2 gas evolution was enhanced, compared with bulk car-
bon nitride, with a turnover number exceeding 6.5 after 25 h of reaction. In another
report, SBA-15 was employed as a hard template to cast an ordered nanostructure
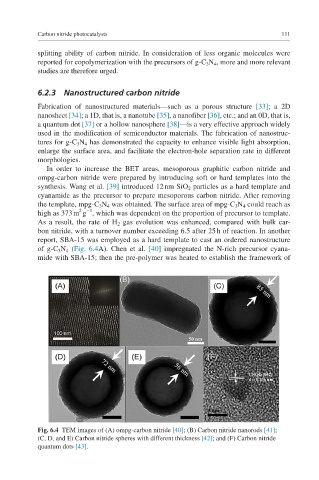
of g-C 3 N 4 (Fig. 6.4A). Chen et al. [40] impregnated the N-rich precursor cyana-
mide with SBA-15; then the pre-polymer was heated to establish the framework of
Fig. 6.4 TEM images of (A) ompg-carbon nitride [40]; (B) Carbon nitride nanorods [41];
(C, D, and E) Carbon nitride spheres with different thickness [42]; and (F) Carbon nitride
quantum dots [43].