Page 154 - Multifunctional Photocatalytic Materials for Energy
P. 154
140 Multifunctional Photocatalytic Materials for Energy
Table 7.2 Continued
2
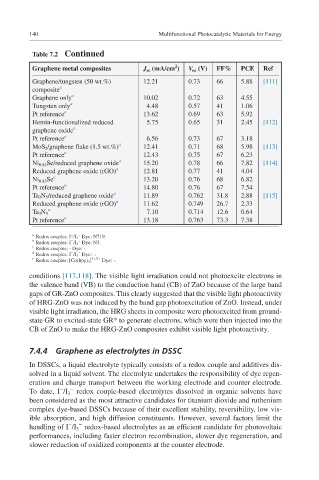
Graphene metal composites J sc (mA/cm ) V oc (V) FF% PCE Ref
Graphene/tungsten (50 wt.%) 12.21 0.73 66 5.88 [111]
composite a
Graphene only a 10.02 0.72 63 4.55
Tungsten only a 4.48 0.57 41 1.06
Pt reference a 13.62 0.69 63 5.92
Hemin-functionalized reduced 5.75 0.65 31 2.45 [112]
graphene oxide a
Pt reference a 6.56 0.73 67 3.18
MoS 2 /graphene flake (1.5 wt.%) a 12.41 0.71 68 5.98 [113]
Pt reference a 12.43 0.75 67 6.23
Ni 0.85 Se/reduced graphene oxide a 15.20 0.78 66 7.82 [114]
Reduced graphene oxide (rGO) a 12.81 0.77 41 4.04
Ni 0.85 Se a 13.20 0.76 68 6.82
Pt reference a 14.80 0.76 67 7.54
Ta 3 N 5 /reduced graphene oxide a 11.89 0.762 31.8 2.88 [115]
Reduced graphene oxide (rGO) a 11.62 0.749 26.7 2.33
a
Ta 3 N 5 7.10 0.714 12.6 0.64
Pt reference a 13.18 0.763 73.3 7.38
a Redox couples: I /I 3 Dye: N719.
−
−
b Redox couples: I /I 3 Dye: N3.
−
−
c Redox couples: - Dye: -.
d Redox couples: I /I 3 Dye: -.
−
−
e Redox couples: [Co(bpy) 3 ] 3+/2+ Dye: -.
conditions [117,118]. The visible light irradiation could not photoexcite electrons in
the valence band (VB) to the conduction band (CB) of ZnO because of the large band
gaps of GR-ZnO composites. This clearly suggested that the visible light photoactivity
of HRG-ZnO was not induced by the band gap photoexcitation of ZnO. Instead, under
visible light irradiation, the HRG sheets in composite were photoexcited from ground-
state GR to excited-state GR* to generate electrons, which were then injected into the
CB of ZnO to make the HRG-ZnO composites exhibit visible light photoactivity.
7.4.4 Graphene as electrolytes in DSSC
In DSSCs, a liquid electrolyte typically consists of a redox couple and additives dis-
solved in a liquid solvent. The electrolyte undertakes the responsibility of dye regen-
eration and charge transport between the working electrode and counter electrode.
−
−
To date, I /I 3 redox couple-based electrolytes dissolved in organic solvents have
been considered as the most attractive candidates for titanium dioxide and ruthenium
complex dye-based DSSCs because of their excellent stability, reversibility, low vis-
ible absorption, and high diffusion constituents. However, several factors limit the
−
−
handling of I /I 3 redox-based electrolytes as an efficient candidate for photovoltaic
performances, including faster electron recombination, slower dye regeneration, and
slower reduction of oxidized components at the counter electrode.