Page 151 - Multifunctional Photocatalytic Materials for Energy
P. 151
Graphene-based nanomaterials for solar cells 137
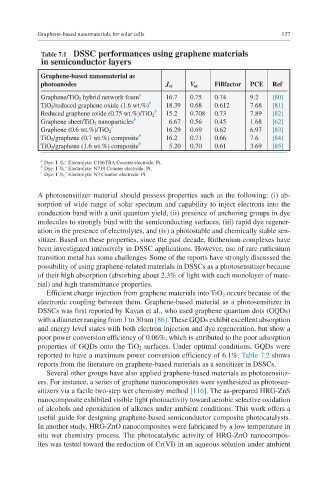
Table 7.1 DSSC performances using graphene materials
in semiconductor layers
Graphene-based nanomaterial as
photoanodes J sc V oc Fillfactor PCE Ref
Graphene/TiO 2 hybrid network foam a 16.7 0.75 0.74 9.2 [80]
TiO 2 /reduced graphene oxide (1.6 wt.%) b 18.39 0.68 0.612 7.68 [81]
b
Reduced graphene oxide (0.75 wt.%)/TiO 2 15.2 0.708 0.73 7.89 [82]
Graphene sheet/TiO 2 nanoparticles b 6.67 0.56 0.45 1.68 [62]
c
Graphene (0.6 wt.%)/TiO 2 16.29 0.69 0.62 6.97 [83]
TiO 2 /graphene (0.7 wt.%) composite b 16.2 0.71 0.66 7.6 [84]
TiO 2 /graphene (1.6 wt.%) composite b 5.20 0.70 0.61 3.69 [85]
−
−
a Dye: I /I 3 Electrolyte: C106TBA Counter electrode: Pt.
b Dye: I /I 3 Electrolyte: N719 Counter electrode: Pt.
−
−
−
−
c Dye: I /I 3 Electrolyte: N3 Counter electrode: Pt.
A photosensitizer material should possess properties such as the following: (i) ab-
sorption of wide range of solar spectrum and capability to inject electrons into the
conduction band with a unit quantum yield, (ii) presence of anchoring groups in dye
molecules to strongly bind with the semiconducting surfaces, (iii) rapid dye regener-
ation in the presence of electrolytes, and (iv) a photostable and chemically stable sen-
sitizer. Based on these properties, since the past decade, Ruthenium complexes have
been investigated intensively in DSSC applications. However, use of rare ruthenium
transition metal has some challenges. Some of the reports have strongly discussed the
possibility of using graphene-related materials in DSSCs as a photosensitizer because
of their high absorption (absorbing about 2.3% of light with each monolayer of mate-
rial) and high transmittance properties.
Efficient charge injection from graphene materials into TiO 2 occurs because of the
electronic coupling between them. Graphene-based material as a photosensitizer in
DSSCs was first reported by Kavan et al., who used graphene quantum dots (GQDs)
with a diameter ranging from 1 to 30 nm [86]. These GQDs exhibit excellent absorption
and energy level states with both electron injection and dye regeneration, but show a
poor power conversion efficiency of 0.06%, which is attributed to the poor adsorption
properties of GQDs onto the TiO 2 surfaces. Under optimal conditions, GQDs were
reported to have a maximum power conversion efficiency of 6.1%. Table 7.2 shows
reports from the literature on graphene-based materials as a sensitizer in DSSCs.
Several other groups have also applied graphene-based materials as photosensitiz-
ers. For instance, a series of graphene nanocomposites were synthesized as photosen-
sitizers via a facile two-step wet chemistry method [116]. The as-prepared HRG-ZnS
nanocomposite exhibited visible light photoactivity toward aerobic selective oxidation
of alcohols and epoxidation of alkenes under ambient conditions. This work offers a
useful guide for designing graphene-based semiconductor composite photocatalysts.
In another study, HRG-ZnO nanocomposites were fabricated by a low temperature in
situ wet chemistry process. The photocatalytic activity of HRG-ZnO nanocompos-
ites was tested toward the reduction of Cr(VI) in an aqueous solution under ambient