Page 18 - Multifunctional Photocatalytic Materials for Energy
P. 18
Metal oxide powder photocatalysts 7
(more negative than) the VB of the semiconductor, whereas the potential of the electron
acceptor species should be located below (more positive than) the CB of the semicon-
ductor. Interfacial electron transfer processes are then initiating subsequent redox reac-
tions to form free radicals for primary and secondary reactions. The free radicals formed
(such as hydroxyl and super oxide radicals) will be used as strong oxidizing agents for
decomposing or degrading the organic pollutants, and so on.
This chapter is designed to introduce and fully explain the background, theory, and
concepts necessary for understanding metal oxides (TiO 2 , ZnO, SnO 2 , CeO 2, etc.) as
photocatalysts [3–10]. In this chapter, emphasis is placed on the optical and electronic
properties of the metal oxides, which will be described with the use of the band model,
and so on. Later, various examples are used to discuss photocatalysis in detail.
2.3 Fundamentals of photocatalysis
Photocatalysis, in short, is defined as “acceleration of a reaction in presence of a suit-
able catalyst and appropriate light.” A catalyst is not altered or used up during a chem-
ical reaction, and it accelerates the rate of a reaction by lowering the activation energy.
It involves photosensitization, which is a process by which a photochemical reaction
takes place in one molecular unit as a result of the initial absorption of light energy by
another molecular unit, called the photosensitized. Photocatalysis assists in forming
strong reducing and oxidizing agents, which helps in breaking down organic matter
into CO 2 and H 2 O in the presence of light, a photocatalyst, and water [4,11–14].
2.3.1 Mechanism
When photocatalysts (such as TiO 2 , ZnO, SnO 2 , CeO 2 , etc.) absorb suitable light, a
pair of electrons and holes in the CB and VB are produced. The electrons of the VB
of metal oxides become excited when irradiated by light. The excess energy of the
excited electrons promotes the electron to the CB of metal oxides. Therefore negative-
+
−
electron (e ) and positive-hole (h ) pairs are created. This stage is referred to as the
semiconductor's “photo-excitation” state. The energy difference between the VB and
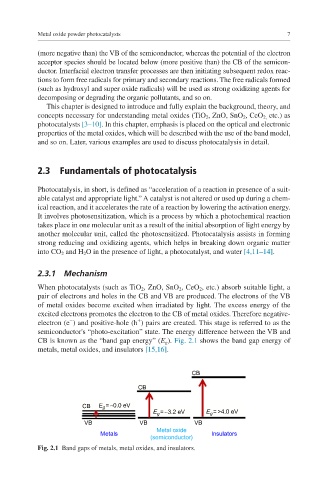
CB is known as the “band gap energy” (E g ). Fig. 2.1 shows the band gap energy of
metals, metal oxides, and insulators [15,16].
CB
CB
CB E g = ∼0.0 eV
E = ∼3.2 eV E =>4.0 eV
g
g
VB VB VB
Metal oxide
Metals Insulators
(semiconductor)
Fig. 2.1 Band gaps of metals, metal oxides, and insulators.