Page 20 - Multifunctional Photocatalytic Materials for Energy
P. 20
Metal oxide powder photocatalysts 9
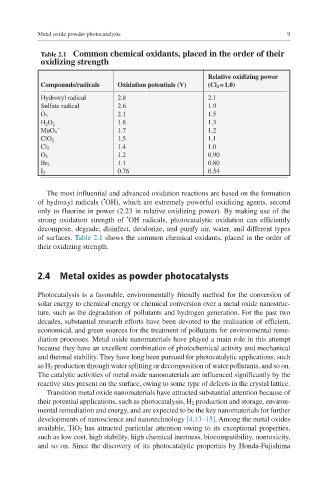
Table 2.1 Common chemical oxidants, placed in the order of their
oxidizing strength
Relative oxidizing power
Compounds/radicals Oxidation potentials (V) (Cl 2 = 1.0)
Hydroxyl radical 2.8 2.1
Sulfate radical 2.6 1.9
O 3 2.1 1.5
H 2 O 2 1.8 1.3
−
MnO 4 1.7 1.2
ClO 2 1.5 1.1
Cl 2 1.4 1.0
O 2 1.2 0.90
Br 2 1.1 0.80
I 2 0.76 0.54
The most influential and advanced oxidation reactions are based on the formation
•
of hydroxyl radicals ( OH), which are extremely powerful oxidizing agents, second
only to fluorine in power (2.23 in relative oxidizing power). By making use of the
•
strong oxidation strength of OH radicals, photocatalytic oxidation can efficiently
decompose, degrade, disinfect, deodorize, and purify air, water, and different types
of surfaces. Table 2.1 shows the common chemical oxidants, placed in the order of
their oxidizing strength.
2.4 Metal oxides as powder photocatalysts
Photocatalysis is a favorable, environmentally friendly method for the conversion of
solar energy to chemical energy or chemical conversion over a metal oxide nanostruc-
ture, such as the degradation of pollutants and hydrogen generation. For the past two
decades, substantial research efforts have been devoted to the realization of efficient,
economical, and green sources for the treatment of pollutants for environmental reme-
diation processes. Metal oxide nanomaterials have played a main role in this attempt
because they have an excellent combination of photochemical activity and mechanical
and thermal stability. They have long been pursued for photocatalytic applications, such
as H 2 production through water splitting or decomposition of water pollutants, and so on.
The catalytic activities of metal oxide nanomaterials are influenced significantly by the
reactive sites present on the surface, owing to some type of defects in the crystal lattice.
Transition metal oxide nanomaterials have attracted substantial attention because of
their potential applications, such as photocatalysis, H 2 production and storage, environ-
mental remediation and energy, and are expected to be the key nanomaterials for further
developments of nanoscience and nanotechnology [4,13–15]. Among the metal oxides
available, TiO 2 has attracted particular attention owing to its exceptional properties,
such as low cost, high stability, high chemical inertness, biocompatibility, nontoxicity,
and so on. Since the discovery of its photocatalytic properties by Honda-Fujishima