Page 202 - Multifunctional Photocatalytic Materials for Energy
P. 202
188 Multifunctional Photocatalytic Materials for Energy
transformation are acceptable only at very high temperatures. The value of the Gibbs
free energy change of water dissociation at standard conditions (237 kJ/mol H 2 ) corre-
sponds to a minimum applied voltage of +1.23 V or an energy requirement of 1.23 eV,
according to
DG =- n F×× DE o with n = 2, F = Faradaysconstant, DE = voltage differeence
’
o
o
In other words, in order to start water splitting using an electrochemical cell, a min-
imum necessary cell voltage of +1.23 V is required to move one electron.
In the other way, water splitting is thermodynamically feasible using light radia-
tions with wavelengths shorter than 1100 nm:
/
(
BandgapeV) = ( h 6 626. ×10 -34 Js) × ( c 30/ . ×10 8 ms) × 10 9 nm / m
l ( nm) 1.6610× - 19 J/ eV
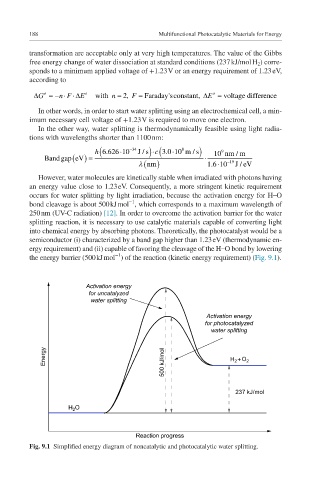
However, water molecules are kinetically stable when irradiated with photons having
an energy value close to 1.23 eV. Consequently, a more stringent kinetic requirement
occurs for water splitting by light irradiation, because the activation energy for HO
−1
bond cleavage is about 500 kJ mol , which corresponds to a maximum wavelength of
250 nm (UV-C radiation) [12]. In order to overcome the activation barrier for the water
splitting reaction, it is necessary to use catalytic materials capable of converting light
into chemical energy by absorbing photons. Theoretically, the photocatalyst would be a
semiconductor (i) characterized by a band gap higher than 1.23 eV (thermodynamic en-
ergy requirement) and (ii) capable of favoring the cleavage of the HO bond by lowering
−1
the energy barrier (500 kJ mol ) of the reaction (kinetic energy requirement) (Fig. 9.1).
Activation energy
for uncatalyzed
water splitting
Activation energy
for photocatalyzed
water splitting
Energy 500 kJ/mol H +O 2
2
237 kJ/mol
H 2 O
Reaction progress
Fig. 9.1 Simplified energy diagram of noncatalytic and photocatalytic water splitting.