Page 66 - Multifunctional Photocatalytic Materials for Energy
P. 66
Energy band engineering of metal oxide for enhanced visible light absorption 55
4.3.2 Solar energy conversion to chemical fuels
Photocatalysis has numerous applications, including organic pollutants' degradation,
self-surface clearance, water treatment, fuel generation, and so on. Using solar en-
ergy to produce chemical fuels (H 2 by splitting water and hydrocarbons by reducing
CO 2 ) is the most attractive application, however, because it provides a way to meet
the world's steadily increasing energy demands and also addresses climate changes
caused by the emission of greenhouse gases.
Hydrogen is a clean, high-density energy because when it is burned with ox-
ygen, water is the only product. Commercial hydrogen is usually produced by the
steam reforming of natural gas, which requires a processing temperature greater
than 1000 K [31]. Initiating water splitting thermodynamically requires an energy in-
−1
put of 237.18 kJ mol . Solar energy is an attractive and renewable energy source.
In view of the electrochemical potential, the minimum band gap of a semiconductor
for photocatalytic water splitting should be 1.23 eV plus the required overpotentials.
Meanwhile, the CB position must be more negative than the H 2 generation potential
and the VB more positive than the O 2 generation potential to allow overall water split-
ting. Numerous photocatalysts have been explored and developed for this purpose. We
do not provide the details on this topic, but related discussions appear throughout the
chapter.
Another strategy to generate solar fuel is the photocatalytic conversion of CO 2 to
hydrocarbons. This actually comes from the natural photosynthesis process, where
green plants convert CO 2 under sunlight to O 2 and hydrocarbons. This concept was
experimentally confirmed by Fujishima and coworkers in 1970s [1,2]. This approach
is currently even more important given the heavy carbon emissions and the climate
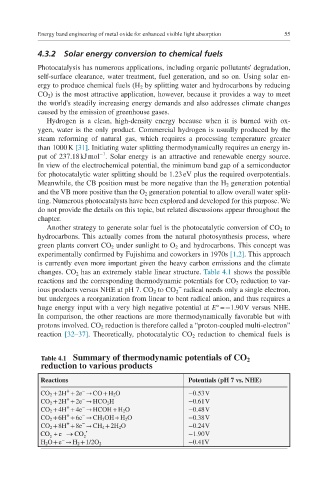
changes. CO 2 has an extremely stable linear structure. Table 4.1 shows the possible
reactions and the corresponding thermodynamic potentials for CO 2 reduction to var-
−
ious products versus NHE at pH 7. CO 2 to CO 2 radical needs only a single electron,
but undergoes a reorganization from linear to bent radical anion, and thus requires a
huge energy input with a very high negative potential at E° = −1.90 V versus NHE.
In comparison, the other reactions are more thermodynamically favorable but with
protons involved. CO 2 reduction is therefore called a “proton-coupled multi- electron”
reaction [32–37]. Theoretically, photocatalytic CO 2 reduction to chemical fuels is
Table 4.1 Summary of thermodynamic potentials of CO 2
reduction to various products
Reactions Potentials (pH 7 vs. NHE)
+
−
CO 2 + 2H + 2e → CO + H 2 O −0.53 V
+
−
CO 2 + 2H + 2e → HCO 2 H −0.61 V
+
−
CO 2 + 4H + 4e → HCOH + H 2 O −0.48 V
−
+
CO 2 + 6H + 6e → CH 3 OH + H 2 O −0.38 V
−
+
CO 2 + 8H + 8e → CH 4 + 2H 2 O −0.24 V
CO + e - ® CO 2 - • −1.90 V
2
−
H 2 O + e → H 2 + 1/2O 2 −0.41V