Page 68 - Multifunctional Photocatalytic Materials for Energy
P. 68
Energy band engineering of metal oxide for enhanced visible light absorption 57
–2
•−
CO 2 /CO 2
CO 2 /HCOOH
Potential vs. NHE (V) at pH = 1 0 1 hv e – H 2 O/H 2 Photoanode H 2 O C x H y O z Cathode
–1
CO 2 /CO
–
e
CB
CO 2 /HCOH
CO 2 /CH 3 OH
CO 2 /CH 4
+
h
CO 2
3 2 VB Semiconductor O 2 H 2 O
(A) (B)
H 2 O O 2 H 2 O C x H y O z
Photocathode Anode Photoanode Photocathode
CO 2
H 2 O
H 2 O
C x H y O z
O 2
CO 2
(C) (D)
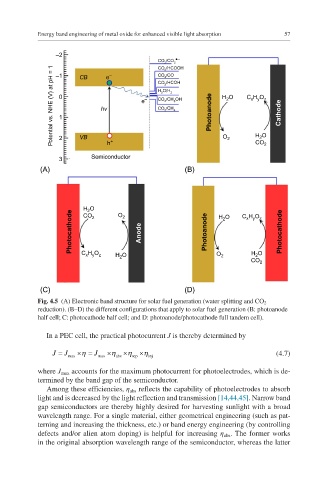
Fig. 4.5 (A) Electronic band structure for solar fuel generation (water splitting and CO 2
reduction). (B–D) the different configurations that apply to solar fuel generation (B: photoanode
half cell; C: photocathode half cell; and D: photoanode/photocathode full tandem cell).
In a PEC cell, the practical photocurrent J is thereby determined by
h
J = J max ´ = J max ´h abs ´h sep ´h inj (4.7)
where J max accounts for the maximum photocurrent for photoelectrodes, which is de-
termined by the band gap of the semiconductor.
Among these efficiencies, η abs reflects the capability of photoelectrodes to absorb
light and is decreased by the light reflection and transmission [14,44,45]. Narrow band
gap semiconductors are thereby highly desired for harvesting sunlight with a broad
wavelength range. For a single material, either geometrical engineering (such as pat-
terning and increasing the thickness, etc.) or band energy engineering (by controlling
defects and/or alien atom doping) is helpful for increasing η abs . The former works
in the original absorption wavelength range of the semiconductor, whereas the latter