Page 72 - Multifunctional Photocatalytic Materials for Energy
P. 72
Energy band engineering of metal oxide for enhanced visible light absorption 61
W [6,46,53]. Large overpotentials are required for BVO photoanodes because of their
poor water oxidation kinetics and their lower conduction band, as compared to the H 2
evolution potential [54,55]. A co-catalyst such as Co-Pi is needed to accelerate an ox-
2
ygen evolution reaction. A high photocurrent was reported to be 2.73 mA/cm at 0.6 V
versus RHE on the nanoporous BiVO 4 photoanode with a FeOOH/NiOOH dual layer
as the OER catalyst [46]. Recently, a more efficient BVO modified by electrochemi-
2
cal reduction was reported to have a photocurrent up to 3.2 mA/cm with CoBi as the
surface cocatalyst [56].
4.4.2.4 Cu-based oxides
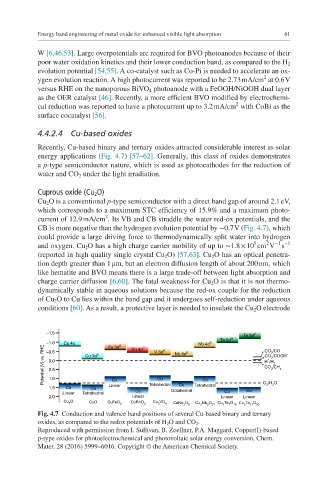
Recently, Cu-based binary and ternary oxides attracted considerable interest as solar
energy applications (Fig. 4.7) [57–62]. Generally, this class of oxides demonstrates
a p-type semiconductor nature, which is used as photocathodes for the reduction of
water and CO 2 under the light irradiation.
Cuprous oxide (Cu 2 O)
Cu 2 O is a conventional p-type semiconductor with a direct band gap of around 2.1 eV,
which corresponds to a maximum STC efficiency of 15.9% and a maximum photo-
2
current of 12.9 mA/cm . Its VB and CB straddle the water red-ox potentials, and the
CB is more negative than the hydrogen evolution potential by −0.7 V (Fig. 4.7), which
could provide a large driving force to thermodynamically split water into hydrogen
2
5
−1 −1
and oxygen. Cu 2 O has a high charge carrier mobility of up to ∼1.8 × 10 cm V s
(reported in high quality single crystal Cu 2 O) [57,63]. Cu 2 O has an optical penetra-
tion depth greater than 1 μm, but an electron diffusion length of about 200 nm, which
like hematite and BVO means there is a large trade-off between light absorption and
charge carrier diffusion [6,60]. The fatal weakness for Cu 2 O is that it is not thermo-
dynamically stable in aqueous solutions because the red-ox couple for the reduction
of Cu 2 O to Cu lies within the band gap and it undergoes self-reduction under aqueous
conditions [60]. As a result, a protective layer is needed to insulate the Cu 2 O electrode
–1.5 Ta 5d 0
Ta 5d 0 CO /CO
Potential (V) vs. RHE –1.0 Cu 3d 9 V 3d Nb 4d 0 CO /COOH –
Cu 4s
0
Nb 4d
5
Fe 3d
7
Rh 4d
0
–0.5
2
2
0.0
+
H /H
2
CO /CH
0.5
2
4
1.0
Cu
Cu
Cu
Cu
2
2
1.5 Cu Cu Linear Tetrahedral Octahedral Tetrahedral Cu O /H O
Linear Tetrahedral Cu Cu
2.0 Linear Linear Linear
Cu O CuO CuFeO 2 CuRhO 2 Cu VO 4 CuNb O 8 Cu Nb O 21 Cu Ta O 19 Cu Ta O 30
2
3
7
3
5
11
8
2
3
Fig. 4.7 Conduction and valence band positions of several Cu-based binary and ternary
oxides, as compared to the redox potentials of H 2 O and CO 2 .
Reproduced with permission from I. Sullivan, B. Zoellner, P.A. Maggard, Copper(I)-based
p-type oxides for photoelectrochemical and photovoltaic solar energy conversion, Chem.
Mater. 28 (2016) 5999–6016. Copyright © the American Chemical Society.