Page 75 - Multifunctional Photocatalytic Materials for Energy
P. 75
64 Multifunctional Photocatalytic Materials for Energy
photocatalytic activity in the broad light region. However, this is a unique case for
doping-induced band gap narrowing, and more investigation may be required to un-
derstand the underlying mechanism.
4.5.2 Solid solution effects in multiple cation oxides
Many metal oxides have a wide band gap and limited visible light absorption. As
previously discussed, doping anions leads to possible visible light absorption by in-
troducing internal energy levels. Metal oxide semiconductors with metal cations that
have occupied d or s orbitals, can absorb large portions of visible light such like Fe 2 O 3 .
The coupling between the filled cation s and O 2p states, however, generally results
in the formation of an indirect band gap semiconductor [51]. Its band-edge optical
absorption varies with the square root of the photon energy (Eq. 4.5), which requires
a thicker film for more light absorption and decreases the carrier extraction efficiency.
Another issue facing these metal oxides is poor charge carrier transport capability
because their conductivity is dominated by a small polaron [51]. Overall, the trade-off
between light absorption and charge carrier transport (η abs × η sep ) is a real challenge for
the use of these kinds of metal oxide semiconductors as photocatalytic applications.
To overcome the intrinsic limitations of binary metal oxides, combining multiple
cations to form functionalized ternary or more oxides is attractive for the next gener-
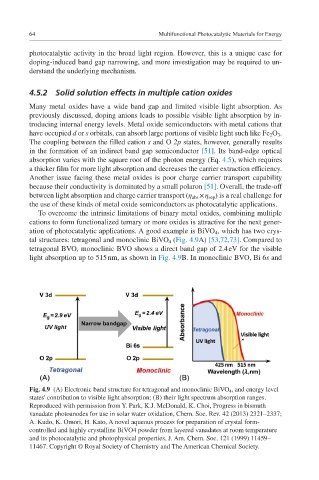
ation of photocatalytic applications. A good example is BiVO 4 , which has two crys-
tal structures: tetragonal and monoclinic BiVO 4 (Fig. 4.9A) [53,72,73]. Compared to
tetragonal BVO, monoclinic BVO shows a direct band gap of 2.4 eV for the visible
light absorption up to 515 nm, as shown in Fig. 4.9B. In monoclinic BVO, Bi 6s and
Fig. 4.9 (A) Electronic band structure for tetragonal and monoclinic BiVO 4 , and energy level
states' contribution to visible light absorption; (B) their light spectrum absorption ranges.
Reproduced with permission from Y. Park, K.J. McDonald, K. Choi, Progress in bismuth
vanadate photoanodes for use in solar water oxidation, Chem. Soc. Rev. 42 (2013) 2321–2337;
A. Kudo, K. Omori, H. Kato, A novel aqueous process for preparation of crystal form-
controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature
and its photocatalytic and photophysical properties, J. Am. Chem. Soc. 121 (1999) 11459–
11467. Copyright © Royal Society of Chemistry and The American Chemical Society.