Page 504 - Book Hosokawa Nanoparticle Technology Handbook
P. 504
APPLICATIONS 14 BARIUM TITANATE NANOPARTICLES SYNTHESIS
50 nm 50 nm
(a) (b)
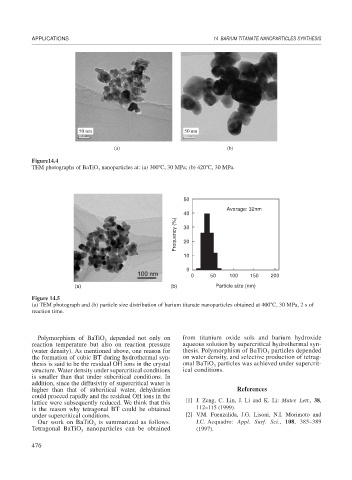
Figure14.4
TEM photographs of BaTiO nanoparticles at: (a) 300 C, 30 MPa; (b) 420 C, 30 MPa.
3
50
Average: 32nm
40
Frequency (%) 30
20
10
0
0 50 100 150 200
(a) (b) Particle size (nm)
Figure 14.5
(a) TEM photograph and (b) particle size distribution of barium titanate nanoparticles obtained at 400 C, 30 MPa, 2 s of
reaction time.
Polymorphism of BaTiO depended not only on from titanium oxide sols and barium hydroxide
3
reaction temperature but also on reaction pressure aqueous solution by supercritical hydrothermal syn-
(water density). As mentioned above, one reason for thesis. Polymorphism of BaTiO particles depended
3
the formation of cubic BT during hydrothermal syn- on water density, and selective production of tetrag-
thesis is said to be the residual OH ions in the crystal onal BaTiO particles was achieved under supercrit-
3
structure. Water density under supercritical conditions ical conditions.
is smaller than that under subcritical conditions. In
addition, since the diffusivity of supercritical water is
higher than that of subcritical water, dehydration References
could proceed rapidly and the residual OH ions in the
lattice were subsequently reduced. We think that this [1] J. Zeng, C. Lin, J. Li and K. Li: Mater. Lett., 38,
is the reason why tetragonal BT could be obtained 112–115 (1999).
under supercritical conditions. [2] V.M. Fuenzalida, J.G. Lisoni, N.I. Morimoto and
Our work on BaTiO is summarized as follows. J.C. Acquadro: Appl. Surf. Sci., 108, 385–389
3
Tetragonal BaTiO nanoparticles can be obtained (1997).
3
476