Page 482 - New Trends in Eco efficient and Recycled Concrete
P. 482
432 New Trends in Eco-efficient and Recycled Concrete
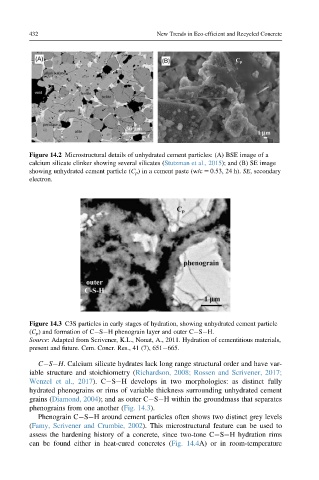
Figure 14.2 Microstructural details of unhydrated cement particles: (A) BSE image of a
calcium silicate clinker showing several silicates (Stutzman et al., 2015); and (B) SE image
showing unhydrated cement particle (C p ) in a cement paste (w/c 5 0.53, 24 h). SE, secondary
electron.
Figure 14.3 C3S particles in early stages of hydration, showing unhydrated cement particle
(C p ) and formation of C S H phenograin layer and outer C S H.
Source: Adapted from Scrivener, K.L., Nonat, A., 2011. Hydration of cementitious materials,
present and future. Cem. Concr. Res., 41 (7), 651 665.
C S H. Calcium silicate hydrates lack long range structural order and have var-
iable structure and stoichiometry (Richardson, 2008; Rossen and Scrivener, 2017;
Wenzel et al., 2017). C S H develops in two morphologies: as distinct fully
hydrated phenograins or rims of variable thickness surrounding unhydrated cement
grains (Diamond, 2004); and as outer C S H within the groundmass that separates
phenograins from one another (Fig. 14.3).
Phenograin C S H around cement particles often shows two distinct grey levels
(Famy, Scrivener and Crumbie, 2002). This microstructural feature can be used to
assess the hardening history of a concrete, since two-tone C S Hhydration rims
can be found either in heat-cured concretes (Fig. 14.4A) or in room-temperature