Page 211 - Numerical Methods for Chemical Engineering
P. 211
200 4 Initial value problems
1
2
2
1
2 1
1
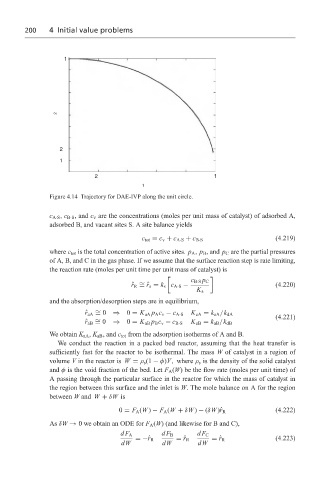
Figure 4.14 Trajectory for DAE-IVP along the unit circle.
c A·S , c B·S , and c v are the concentrations (moles per unit mass of catalyst) of adsorbed A,
adsorbed B, and vacant sites S. A site balance yields
c tot = c v + c A·S + c B·S (4.219)
where c tot is the total concentration of active sites. p A , p B , and p C are the partial pressures
of A, B, and C in the gas phase. If we assume that the surface reaction step is rate limiting,
the reaction rate (moles per unit time per unit mass of catalyst) is
c B·S p C
∼
ˆ r R = ˆ r s = k s c A·S − (4.220)
K s
and the absorption/desorption steps are in equilibrium,
∼
ˆ r aA = 0 ⇒ 0 = K aA p A c v − c A·S K aA = k aA /k dA
(4.221)
∼
ˆ r aB = 0 ⇒ 0 = K aB p B c v − c B·S K aB = k aB /k dB
We obtain K aA , K aB , and c tot from the adsorption isotherms of A and B.
We conduct the reaction in a packed bed reactor, assuming that the heat transfer is
sufficiently fast for the reactor to be isothermal. The mass W of catalyst in a region of
volume V in the reactor is W = ρ s (1 − φ)V, where ρ s is the density of the solid catalyst
and φ is the void fraction of the bed. Let F A (W) be the flow rate (moles per unit time) of
A passing through the particular surface in the reactor for which the mass of catalyst in
the region between this surface and the inlet is W. The mole balance on A for the region
between W and W + δW is
(4.222)
0 = F A (W) − F A (W + δW) − (δW)ˆ r R
As δW → 0 we obtain an ODE for F A (W) (and likewise for B and C),
dF A dF B dF C
=−ˆ r R = ˆ r R = ˆ r R (4.223)
dW dW dW