Page 329 - Numerical Methods for Chemical Engineering
P. 329
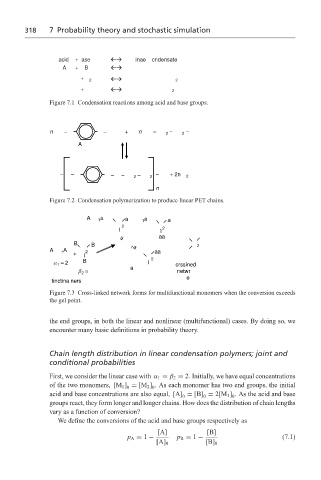
318 7 Probability theory and stochastic simulation
acid + ase inae cndensate
A + B
+ 2 2
+ 2
Figure 7.1 Condensation reactions among acid and base groups.
n − − + n − 2 − 2 −
A
− − − − 2 − 2 − + 2n 2
n
Figure 7.2 Condensation polymerization to produce linear PET chains.
A 1 a a 1 a a
2
2
aa
a
B B 2
A 1 A + 2 1 a aa
α = 2 B 2 crssined
1
β = a netwr
2
e
tinctina ners
Figure 7.3 Cross-linked network forms for multifunctional monomers when the conversion exceeds
the gel point.
the end groups, in both the linear and nonlinear (multifunctional) cases. By doing so, we
encounter many basic definitions in probability theory.
Chain length distribution in linear condensation polymers; joint and
conditional probabilities
First, we consider the linear case with α 1 = β 2 = 2. Initially, we have equal concentrations
of the two monomers, [M 1 ] = [M 2 ] . As each monomer has two end groups, the initial
0
0
acid and base concentrations are also equal, [A] = [B] = 2[M 1 ] . As the acid and base
0
0
0
groups react, they form longer and longer chains. How does the distribution of chain lengths
vary as a function of conversion?
We define the conversions of the acid and base groups respectively as
[A] [B]
p A = 1 − p B = 1 − (7.1)
[A] 0 [B] 0