Page 317 - Origin and Prediction of Abnormal Formation Pressures
P. 317
286 H.H. RIEKE, G.V. CHILINGAR AND J.O. ROBERTSON JR.
r
o
.m 100
o 80
.-...
.c_ g 60
r
.o_ g 40
a,_
20
t-"
0
c-
100
80
c-
60
O
40
20
0
100 1000 10,000 100,000
Overburden Pressure, psi
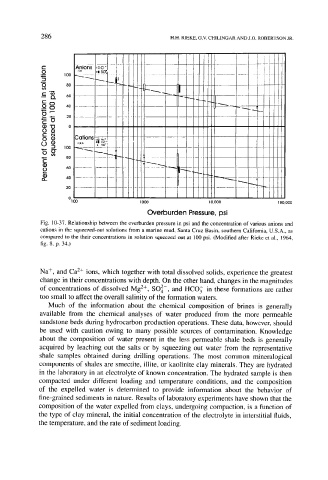
Fig. 10-37. Relationship between the overburden pressure in psi and the concentration of various anions and
cations in the squeezed-out solutions from a marine mud, Santa Cruz Basin, southern California, U.S.A., as
compared to the their concentrations in solution squeezed out at 100 psi. (Modified after Rieke et al., 1964,
fig. 8, p. 34.)
Na +, and Ca 2+ ions, which together with total dissolved solids, experience the greatest
change in their concentrations with depth. On the other hand, changes in the magnitudes
of concentrations of dissolved Mg 2+, SO 2-, and HCO 3 in these formations are rather
too small to affect the overall salinity of the formation waters.
Much of the information about the chemical composition of brines is generally
available from the chemical analyses of water produced from the more permeable
sandstone beds during hydrocarbon production operations. These data, however, should
be used with caution owing to many possible sources of contamination. Knowledge
about the composition of water present in the less permeable shale beds is generally
acquired by leaching out the salts or by squeezing out water from the representative
shale samples obtained during drilling operations. The most common mineralogical
components of shales are smectite, illite, or kaolinite clay minerals. They are hydrated
in the laboratory in an electrolyte of known concentration. The hydrated sample is then
compacted under different loading and temperature conditions, and the composition
of the expelled water is determined to provide information about the behavior of
fine-grained sediments in nature. Results of laboratory experiments have shown that the
composition of the water expelled from clays, undergoing compaction, is a function of
the type of clay mineral, the initial concentration of the electrolyte in interstitial fluids,
the temperature, and the rate of sediment loading.