Page 198 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 198
6.5 Spectra 189
Continuum
0 n 5 ¥
n 5 6
Pfund n 5 5
n 5 4
series
Brackett n 5 3
Paschen series
22
series
n 5 2
Balmer
24 series
26
Energy (eV)
28
210
212
n 5 1
Lyman
214
series
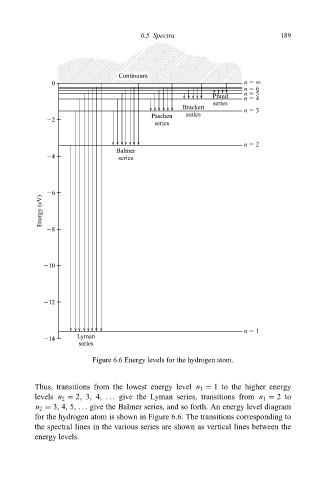
Figure 6.6 Energy levels for the hydrogen atom.
Thus, transitions from the lowest energy level n 1 1 to the higher energy
levels n 2 2, 3, 4, ... give the Lyman series, transitions from n 1 2to
n 2 3, 4, 5, ... give the Balmer series, and so forth. An energy level diagram
for the hydrogen atom is shown in Figure 6.6. The transitions corresponding to
the spectral lines in the various series are shown as vertical lines between the
energy levels.