Page 194 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 194
6.4 Atomic orbitals 185
a 0 D 10 0.60
0.50
0.40
0.30
0.20
0.10
0.00 r/a 0
0 2 4 6 8 10 12 14 16 18 20
a D 2l 0.20
0
0.15
0.10
0.05
0.10 D 20
D 21
0.00 r/a 0
0 2 4 6 8 10 12 14 16 18 20
D 0.12
a 0 3l
0.10
0.08
0.06
D
0.04 30
0.02 D 31
D 32
0.00 r/a 0
0 2 4 6 8 10 12 14 16 18 20
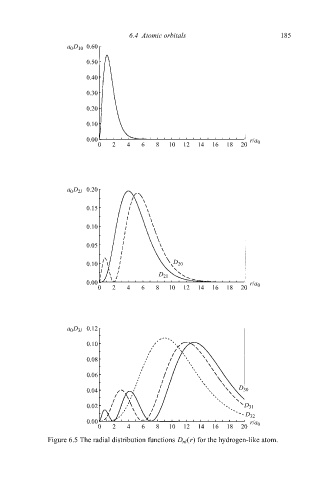
Figure 6.5 The radial distribution functions D nl (r) for the hydrogen-like atom.