Page 52 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 52
2.3 Expectation values of dynamical quantities 43
2
2
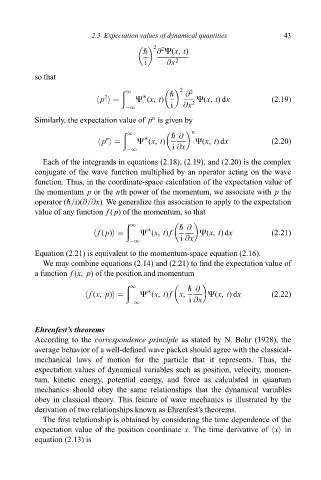
" @ Ø(x, t)
i @x 2
so that
2
1 " @ 2
2
hp i Ø (x, t) Ø(x, t)dx (2:19)
i @x 2
ÿ1
n
Similarly, the expectation value of p is given by
n
1 " @
n
hp i Ø (x, t) Ø(x, t)dx (2:20)
i @x
ÿ1
Each of the integrands in equations (2.18), (2.19), and (2.20) is the complex
conjugate of the wave function multiplied by an operator acting on the wave
function. Thus, in the coordinate-space calculation of the expectation value of
the momentum p or the nth power of the momentum, we associate with p the
operator ("=i)(@=@x). We generalize this association to apply to the expectation
value of any function f ( p) of the momentum, so that
1 " @
hf (p)i Ø (x, t) f Ø(x, t)dx (2:21)
i @x
ÿ1
Equation (2.21) is equivalent to the momentum-space equation (2.16).
We may combine equations (2.14) and (2.21) to ®nd the expectation value of
a function f (x, p) of the position and momentum
1 " @
hf (x, p)i Ø (x, t) fx, Ø(x, t)dx (2:22)
i @x
ÿ1
Ehrenfest's theorems
According to the correspondence principle as stated by N. Bohr (1928), the
average behavior of a well-de®ned wave packet should agree with the classical-
mechanical laws of motion for the particle that it represents. Thus, the
expectation values of dynamical variables such as position, velocity, momen-
tum, kinetic energy, potential energy, and force as calculated in quantum
mechanics should obey the same relationships that the dynamical variables
obey in classical theory. This feature of wave mechanics is illustrated by the
derivation of two relationships known as Ehrenfest's theorems.
The ®rst relationship is obtained by considering the time dependence of the
expectation value of the position coordinate x. The time derivative of hxi in
equation (2.13) is