Page 62 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 62
2.6 Tunneling 53
2.6 Tunneling
As a second example of the application of the Schrodinger equation, we
È
consider the behavior of a particle in the presence of a potential barrier. The
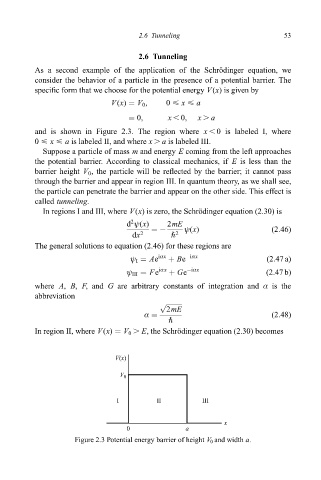
speci®c form that we choose for the potential energy V(x)isgiven by
V(x) V 0 , 0 < x < a
0, x , 0, x . a
and is shown in Figure 2.3. The region where x , 0 is labeled I, where
0 < x < a is labeled II, and where x . a is labeled III.
Suppose a particle of mass m and energy E coming from the left approaches
the potential barrier. According to classical mechanics, if E is less than the
barrier height V 0 , the particle will be re¯ected by the barrier; it cannot pass
through the barrier and appear in region III. In quantum theory, as we shall see,
the particle can penetrate the barrier and appear on the other side. This effect is
called tunneling.
È
In regions I and III, where V(x) is zero, the Schrodinger equation (2.30) is
2
d ø(x) 2mE
ÿ ø(x) (2:46)
dx 2 " 2
The general solutions to equation (2.46) for these regions are
ø I Ae iáx Be ÿiáx (2:47 a)
ø III Fe iáx Ge ÿiáx (2:47 b)
where A, B, F, and G are arbitrary constants of integration and á is the
abbreviation
p
2mE
á (2:48)
"
In region II, where V(x) V 0 . E, the SchroÈdinger equation (2.30) becomes
V(x)
V 0
I II III
x
0 a
Figure 2.3 Potential energy barrier of height V 0 and width a.