Page 59 - PRINCIPLES OF QUANTUM MECHANICS as Applied to Chemistry and Chemical Physics
P. 59
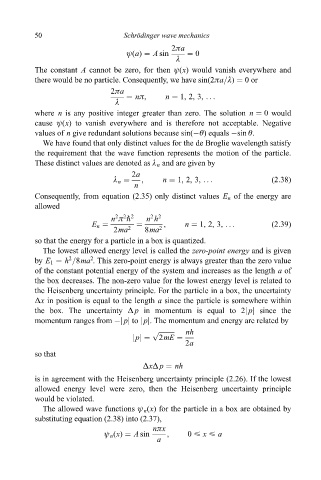
50 Schro Èdinger wave mechanics
2ða
ø(a) A sin 0
ë
The constant A cannot be zero, for then ø(x) would vanish everywhere and
there would be no particle. Consequently, we have sin(2ða=ë) 0or
2ða
nð, n 1, 2, 3, ...
ë
where n is any positive integer greater than zero. The solution n 0 would
cause ø(x) to vanish everywhere and is therefore not acceptable. Negative
values of n give redundant solutions because sin(ÿè) equals ÿsin è.
We have found that only distinct values for the de Broglie wavelength satisfy
the requirement that the wave function represents the motion of the particle.
These distinct values are denoted as ë n and are given by
2a
ë n , n 1, 2, 3, ... (2:38)
n
Consequently, from equation (2.35) only distinct values E n of the energy are
allowed
2 2 2
2 2
n ð " n h
E n , n 1, 2, 3, ... (2:39)
2ma 2 8ma 2
so that the energy for a particle in a box is quantized.
The lowest allowed energy level is called the zero-point energy and is given
2
2
by E 1 h =8ma . This zero-point energy is always greater than the zero value
of the constant potential energy of the system and increases as the length a of
the box decreases. The non-zero value for the lowest energy level is related to
the Heisenberg uncertainty principle. For the particle in a box, the uncertainty
Äx in position is equal to the length a since the particle is somewhere within
the box. The uncertainty Äp in momentum is equal to 2jpj since the
momentum ranges from ÿjpj to jpj. The momentum and energy are related by
p nh
jpj 2mE
2a
so that
ÄxÄp nh
is in agreement with the Heisenberg uncertainty principle (2.26). If the lowest
allowed energy level were zero, then the Heisenberg uncertainty principle
would be violated.
The allowed wave functions ø n (x) for the particle in a box are obtained by
substituting equation (2.38) into (2.37),
nðx
ø n (x) A sin , 0 < x < a
a