Page 182 - Partition & Adsorption of Organic Contaminants in Environmental Systems
P. 182
SORPTION FROM WATER SOLUTION 173
20
1, 2, 3-Trichlorobenzene (24°C)
(mg/L)
Apparent Solute Solubility 15 0 Lindane (24°C)
10
(µg/L) 10
5 p, p'-DDT (23°C)
0
0 200 400 600
Concentration of Phenylethanoic Acid, X (mg/L)
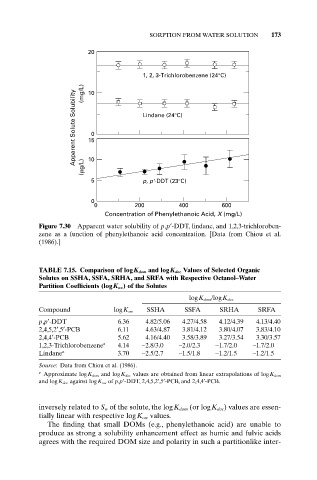
Figure 7.30 Apparent water solubility of p,p¢-DDT, lindane, and 1,2,3-trichloroben-
zene as a function of phenylethanoic acid concentration. [Data from Chiou et al.
(1986).]
TABLE 7.15. Comparison of logK dom and logK doc Values of Selected Organic
Solutes on SSHA, SSFA, SRHA, and SRFA with Respective Octanol–Water
Partition Coefficients (logK ow ) of the Solutes
logK dom/logK doc
Compound logK ow SSHA SSFA SRHA SRFA
p,p¢-DDT 6.36 4.82/5.06 4.27/4.58 4.12/4.39 4.13/4.40
2,4,5,2¢,5¢-PCB 6.11 4.63/4.87 3.81/4.12 3.80/4.07 3.83/4.10
2,4,4¢-PCB 5.62 4.16/4.40 3.58/3.89 3.27/3.54 3.30/3.57
1,2,3-Trichlorobenzene a 4.14 ~2.8/3.0 ~2.0/2.3 ~1.7/2.0 ~1.7/2.0
Lindane a 3.70 ~2.5/2.7 ~1.5/1.8 ~1.2/1.5 ~1.2/1.5
Source: Data from Chiou et al. (1986).
a
Approximate logK dom and logK doc values are obtained from linear extrapolations of logK dom
and logK doc against logK ow of p,p¢-DDT, 2,4,5,2¢,5¢-PCB, and 2,4,4¢-PCB.
inversely related to S w of the solute, the logK dom (or logK doc) values are essen-
tially linear with respective logK ow values.
The finding that small DOMs (e.g., phenylethanoic acid) are unable to
produce as strong a solubility enhancement effect as humic and fulvic acids
agrees with the required DOM size and polarity in such a partitionlike inter-